MICARDIS ®(telmisartan tablets), for oral use
Micardis by
Drug Labeling and Warnings
Micardis by is a Prescription medication manufactured, distributed, or labeled by Praxis, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MICARDIS- telmisartan tablet
Praxis, LLC
----------
MICARDIS ®(telmisartan tablets), for oral use
10 OVERDOSAGE
Limited data are available with regard to overdosage in humans. The most likely manifestation of overdosage with MICARDIS tablets would be hypotension, dizziness and tachycardia; bradycardia could occur from parasympathetic (vagal) stimulation. If symptomatic hypotension should occur, supportive treatment should be instituted. Telmisartan is not removed by hemofiltration and is not dialyzable.
11 DESCRIPTION
MICARDIS is a non-peptide angiotensin II receptor (type AT 1) antagonist.
Telmisartan is chemically described as 4'-[(1,4'-dimethyl-2'-propyl [2,6'-bi-1H-benzimidazol]-1'-yl)methyl]-[1,1'-biphenyl]-2-carboxylic acid. Its empirical formula is C 33H 30N 4O 2, its molecular weight is 514.63, and its structural formula is:
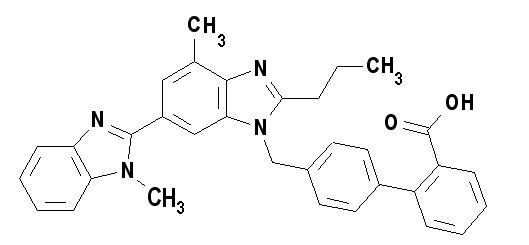
Telmisartan is a white to slightly yellowish solid. It is practically insoluble in water and in the pH range of 3 to 9, sparingly soluble in strong acid (except insoluble in hydrochloric acid), and soluble in strong base.
MICARDIS is available as tablets for oral administration, containing 20 mg, 40 mg or 80 mg of telmisartan. The tablets contain the following inactive ingredients: sodium hydroxide, meglumine, povidone, sorbitol, and magnesium stearate. MICARDIS tablets are hygroscopic and require protection from moisture.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Telmisartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT 1receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Its action is therefore independent of the pathways for angiotensin II synthesis.
There is also an AT 2receptor found in many tissues, but AT 2is not known to be associated with cardiovascular homeostasis. Telmisartan has much greater affinity (>3,000 fold) for the AT 1receptor than for the AT 2receptor.
Blockade of the renin-angiotensin system with ACE inhibitors, which inhibit the biosynthesis of angiotensin II from angiotensin I, is widely used in the treatment of hypertension. ACE inhibitors also inhibit the degradation of bradykinin, a reaction also catalyzed by ACE. Because telmisartan does not inhibit ACE (kininase II), it does not affect the response to bradykinin. Whether this difference has clinical relevance is not yet known. Telmisartan does not bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation.
Blockade of the angiotensin II receptor inhibits the negative regulatory feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and angiotensin II circulating levels do not overcome the effect of telmisartan on blood pressure.
12.2 Pharmacodynamics
In normal volunteers, a dose of telmisartan 80 mg inhibited the pressor response to an intravenous infusion of angiotensin II by about 90% at peak plasma concentrations with approximately 40% inhibition persisting for 24 hours.
Plasma concentration of angiotensin II and plasma renin activity (PRA) increased in a dose-dependent manner after single administration of telmisartan to healthy subjects and repeated administration to hypertensive patients. The once-daily administration of up to 80 mg telmisartan to healthy subjects did not influence plasma aldosterone concentrations. In multiple dose studies with hypertensive patients, there were no clinically significant changes in electrolytes (serum potassium or sodium), or in metabolic function (including serum levels of cholesterol, triglycerides, HDL, LDL, glucose, or uric acid).
In 30 hypertensive patients with normal renal function treated for 8 weeks with telmisartan 80 mg or telmisartan 80 mg in combination with hydrochlorothiazide 12.5 mg, there were no clinically significant changes from baseline in renal blood flow, glomerular filtration rate, filtration fraction, renovascular resistance, or creatinine clearance.
12.3 Pharmacokinetics
Absorption:
Following oral administration, peak concentrations (C
max) of telmisartan are reached in 0.5 to 1 hour after dosing. Food slightly reduces the bioavailability of telmisartan, with a reduction in the area under the plasma concentration-time curve (AUC) of about 6% with the 40 mg tablet and about 20% after a 160 mg dose. The absolute bioavailability of telmisartan is dose dependent. At 40 mg and 160 mg, the bioavailability was 42% and 58%, respectively. The pharmacokinetics of orally administered telmisartan are nonlinear over the dose range 20 mg to 160 mg, with greater than proportional increases of plasma concentrations (C
maxand AUC) with increasing doses. Telmisartan shows bi-exponential decay kinetics with a terminal elimination half-life of approximately 24 hours. Trough plasma concentrations of telmisartan with once daily dosing are about 10% to 25% of peak plasma concentrations. Telmisartan has an accumulation index in plasma of 1.5 to 2.0 upon repeated once daily dosing.
Distribution
Telmisartan is highly bound to plasma proteins (>99.5%), mainly albumin and α
1- acid glycoprotein. Plasma protein binding is constant over the concentration range achieved with recommended doses. The volume of distribution for telmisartan is approximately 500 liters indicating additional tissue binding.
Metabolism and Elimination
Following either intravenous or oral administration of
14C-labeled telmisartan, most of the administered dose (>97%) was eliminated unchanged in feces via biliary excretion; only minute amounts were found in the urine (0.91% and 0.49% of total radioactivity, respectively).
Telmisartan is metabolized by conjugation to form a pharmacologically inactive acyl glucuronide; the glucuronide of the parent compound is the only metabolite that has been identified in human plasma and urine. After a single dose, the glucuronide represents approximately 11% of the measured radioactivity in plasma. The cytochrome P450 isoenzymes are not involved in the metabolism of telmisartan.
Total plasma clearance of telmisartan is >800 mL/min. Terminal half-life and total clearance appear to be independent of dose.
Specific Populations
Renal Insufficiency
No dosage adjustment is necessary in patients with decreased renal function. Telmisartan is not removed from blood by hemofiltration and is not dialyzable
[see Warnings and Precautions
(5.5)and Dosage and Administration
(2.1)]
.
Hepatic Insufficiency
In patients with hepatic insufficiency, plasma concentrations of telmisartan are increased, and absolute bioavailability approaches 100%
[see Warnings and Precautions
(5.4)and Use in Specific Populations
(8.6)]
.
Gender
Plasma concentrations of telmisartan are generally 2 to 3 times higher in females than in males. In clinical trials, however, no significant increases in blood pressure response or in the incidence of orthostatic hypotension were found in women. No dosage adjustment is necessary.
Geriatric Patients
The pharmacokinetics of telmisartan do not differ between the elderly and those younger than 65 years
[see Dosage and Administration
(2.1)]
.
Pediatric Patients
Telmisartan pharmacokinetics have not been investigated in patients <18 years of age.
Drug Interaction Studies
Telmisartan
Ramipril and Ramiprilat: Co-administration of telmisartan 80 mg once daily and ramipril 10 mg once daily to healthy subjects increases steady-state C
maxand AUC of ramipril 2.3- and 2.1-fold, respectively, and C
maxand AUC of ramiprilat 2.4- and 1.5-fold, respectively. In contrast, C
maxand AUC of telmisartan decrease by 31% and 16%, respectively. When co-administering telmisartan and ramipril, the response may be greater because of the possibly additive pharmacodynamic effects of the combined drugs, and also because of the increased exposure to ramipril and ramiprilat in the presence of telmisartan.
Other Drugs: Co-administration of telmisartan did not result in a clinically significant interaction with acetaminophen, amlodipine, glyburide, simvastatin, hydrochlorothiazide, warfarin, or ibuprofen. Telmisartan is not metabolized by the cytochrome P450 system and had no effects in vitroon cytochrome P450 enzymes, except for some inhibition of CYP2C19. Telmisartan is not expected to interact with drugs that inhibit cytochrome P450 enzymes; it is also not expected to interact with drugs metabolized by cytochrome P450 enzymes, except for possible inhibition of the metabolism of drugs metabolized by CYP2C19.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
There was no evidence of carcinogenicity when telmisartan was administered in the diet to mice and rats for up to 2 years. The highest doses administered to mice (1000 mg/kg/day) and rats (100 mg/kg/day) are, on a mg/m 2basis, about 59 and 13 times, respectively, the maximum recommended human dose (MRHD) of telmisartan. These same doses have been shown to provide average systemic exposures to telmisartan >100 times and >25 times, respectively, the systemic exposure in humans receiving the MRHD (80 mg/day).
Genotoxicity assays did not reveal any telmisartan-related effects at either the gene or chromosome level. These assays included bacterial mutagenicity tests with Salmonellaand E. coli(Ames), a gene mutation test with Chinese hamster V79 cells, a cytogenetic test with human lymphocytes, and a mouse micronucleus test.
No drug-related effects on the reproductive performance of male and female rats were noted at 100 mg/kg/day (the highest dose administered), about 13 times, on a mg/m 2basis, the MRHD of telmisartan. This dose in the rat resulted in an average systemic exposure (telmisartan AUC as determined on day 6 of pregnancy) at least 50 times the average systemic exposure in humans at the MRHD (80 mg/day).
14 CLINICAL STUDIES
14.1 Hypertension
The antihypertensive effects of MICARDIS have been demonstrated in six principal placebo-controlled clinical trials, studying a range of 20 to 160 mg; one of these examined the antihypertensive effects of telmisartan and hydrochlorothiazide in combination. The studies involved a total of 1773 patients with mild to moderate hypertension (diastolic blood pressure of 95 to 114 mmHg), 1031 of whom were treated with telmisartan. Following once daily administration of telmisartan, the magnitude of blood pressure reduction from baseline after placebo subtraction was approximately (SBP/DBP) 6-8/6 mmHg for 20 mg, 9-13/6-8 mmHg for 40 mg, and 12-13/7-8 mmHg for 80 mg. Larger doses (up to 160 mg) did not appear to cause a further decrease in blood pressure.
Upon initiation of antihypertensive treatment with telmisartan, blood pressure was reduced after the first dose, with a maximal reduction by about 4 weeks. With cessation of treatment with MICARDIS tablets, blood pressure gradually returned to baseline values over a period of several days to one week. During long-term studies (without placebo control) the effect of telmisartan appeared to be maintained for up to at least one year. The antihypertensive effect of telmisartan is not influenced by patient age, gender, weight, or body mass index. Blood pressure response in black patients (usually a low-renin population) is noticeably less than that in Caucasian patients. This has been true for most, but not all, angiotensin II antagonists and ACE inhibitors.
In a controlled study, the addition of telmisartan to hydrochlorothiazide produced an additional dose-related reduction in blood pressure that was similar in magnitude to the reduction achieved with telmisartan monotherapy. Hydrochlorothiazide also had an added blood pressure effect when added to telmisartan.
The onset of antihypertensive activity occurs within 3 hours after administration of a single oral dose. At doses of 20, 40, and 80 mg, the antihypertensive effect of once daily administration of telmisartan is maintained for the full 24-hour dose interval. With automated ambulatory blood pressure monitoring and conventional blood pressure measurements, the 24-hour trough-to-peak ratio for 40 to 80 mg doses of telmisartan was 70% to 100% for both systolic and diastolic blood pressure. The incidence of symptomatic orthostasis after the first dose in all controlled trials was low (0.04%).
There were no changes in the heart rate of patients treated with telmisartan in controlled trials.
There are no trials of MICARDIS demonstrating reductions in cardiovascular risk in patients with hypertension, but at least one pharmacologically similar drug has demonstrated such benefits.
14.2 Cardiovascular Risk Reduction
Support for use to reduce the risk of cardiovascular events was obtained in a pair of studies. Both enrolled subjects age ≥55 years, at high cardiovascular risk as evidenced by coronary artery disease (75%), diabetes mellitus (27%) accompanied with end-organ damage (e.g., retinopathy, left ventricular hypertrophy, and, in ONTARGET only, macro- or microalbuminuria), stroke (16%), peripheral vascular disease (13%), or transient ischemic attack (4%). Patients without a history of intolerance to ACE inhibitors entered ONTARGET, and those with such a history, usually cough (90%), entered TRANSCEND, but patients with >1+ proteinuria on dipstick were excluded from TRANSCEND. For both ONTARGET and TRANSCEND trials, the primary 4-component composite endpoint was death from cardiovascular causes, myocardial infarction, stroke, and hospitalization for heart failure. The secondary 3-component composite endpoint was death from cardiovascular causes, myocardial infarction, and stroke.
ONTARGET was a randomized, active-controlled, multinational, double-blind study in 25,620 patients who were randomized to telmisartan 80 mg, ramipril 10 mg, or their combination. The population studied was 73% male, 74% Caucasian, 14% Asian, and 57% were 65 years of age or older. Baseline therapy included acetylsalicylic acid (76%), lipid lowering agents (64%), beta-blockers (57%), calcium channel blockers (34%), nitrates (29%), and diuretics (28%). Mean blood pressure at randomization was 134/77 mmHg. The mean duration of follow up was about 4 years and 6 months. During the study, 22.0% (n=1878) of telmisartan patients discontinued the active treatment, compared to 24.4% (n=2095) of ramipril patients and 25.3% (n=2152) of telmisartan/ramipril patients.
TRANSCEND randomized patients to telmisartan 80 mg (n=2954) or placebo (n=2972). The mean duration of follow up was 4 years and 8 months. The population studied was 57% male, 62% Caucasian, 21% Asian, and 60% were 65 years of age or older. Baseline therapy included acetylsalicylic acid (75%), lipid lowering agents (58%), beta-blockers (58%), calcium channel blockers (41%), nitrates (34%) and diuretics (33%). Mean blood pressure at randomization was 135/78 mmHg. During the study, 17.7% (n=523) of telmisartan patients discontinued the active treatment, compared to 19.4% (n=576) of placebo patients.
The results for the TRANSCEND trial are summarized in Table 2, and the results for ONTARGET are summarized in Table 3, below:
| *The primary endpoint was defined as the time to first event. In case of multiple simultaneous events, all individual events were considered; the sum of patients with individual outcomes may exceed the number of patients with composite (primary or secondary) outcomes.
**For individual components of the primary composite endpoints, all events, regardless whether or not they were the first event, were considered. Therefore, they are more than the first events considered for the primary or secondary composite endpoint. |
|||
| Telmisartan vs. Placebo
(n=2954) (n=2972) |
|||
| No. of Events
Telmisartan / Placebo | Hazard Ratio
95% CI | p-value | |
| *Composite of CV death, myocardial infarction, stroke, or hospitalization for heart failure | 465 (15.7%) / 504 (17.0%) | 0.92 (0.81 – 1.05) | 0.2129 |
| *Composite of CV death, myocardial infarction, or stroke | 384 (13.0%) / 440 (14.8%) | 0.87 (0.76 – 1.00) | 0.0483 |
| Individual components of the primary composite endpoint | No. of Events
Telmisartan / Placebo | Hazard Ratio
95% CI | p-value |
| **All non-fatal MI | 114 (3.9%) / 145 (4.9%) | 0.79 (0.62 – 1.01) | 0.0574 |
| **All non-fatal strokes | 112 (3.8%) / 136 (4.6%) | 0.83 (0.64 – 1.06) | 0.1365 |
| Telmisartan vs. Ramipril
(n=8542) (n=8576) |
||
| No. of Events
Telmisartan / Ramipril | Hazard Ratio
97.5% CI |
|
| Composite of CV death, myocardial infarction, stroke, or hospitalization for heart failure | 1423 (16.7%) / 1412 (16.5%) | 1.01 (0.93 – 1.10) |
| Composite of CV death, myocardial infarction, or stroke | 1190 (13.9%) / 1210 (14.1%) | 0.99 (0.90 – 1.08) |
Although the event rates in ONTARGET were similar on telmisartan and ramipril, the results did not unequivocally rule out that MICARDIS may not preserve a meaningful fraction of the effect of ramipril in reducing cardiovascular events. However, the results of both ONTARGET and TRANSCEND do adequately support MICARDIS being more effective than placebo would be in this setting, particularly for the endpoint of time to cardiovascular death, myocardial infarction, or stroke.
In ONTARGET, there was no evidence that combining ramipril and MICARDIS reduced the risk of death from cardiovascular causes, myocardial infarction, stroke, or hospitalization for heart failure greater than ramipril alone; instead, patients who received the combination of ramipril and telmisartan in ONTARGET experienced an increased incidence of clinically important renal dysfunction (e.g., acute renal failure) compared to patients receiving MICARDIS or ramipril alone.
Multiple sub-group analyses did not demonstrate any differences in the 4-component composite primary endpoint based on age, gender, or ethnicity for either ONTARGET or TRANSCEND trial.
16 HOW SUPPLIED/STORAGE AND HANDLING
MICARDIS is available as white or off-white, uncoated tablets containing telmisartan 20 mg, 40 mg, or 80 mg. Tablets are marked with the Boehringer Ingelheim company symbol on one side, and on the other side, with either "50H", "51H", or "52H" for the 20 mg, 40 mg, and 80 mg strengths, respectively. Tablets are provided as follows:
MICARDIS tablets 20 mg are round and individually blister-sealed in cartons of 30 tablets as 3 x 10 cards (NDC: 0597-0039-37).
MICARDIS tablets 40 mg are oblong shaped and individually blister-sealed in cartons of 30 tablets as 3 x 10 cards (NDC: 0597-0040-37).
MICARDIS tablets 80 mg are oblong shaped and individually blister-sealed in cartons of 30 tablets as 3 x 10 cards (NDC: 0597-0041-37).
Storage
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F)[see USP Controlled Room Temperature]. Tablets should not be removed from blisters until immediately before administration.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information)
Pregnancy
Advise female patients of childbearing age about the consequences of exposure to MICARDIS during pregnancy. Discuss treatment options with women planning to become pregnant. Tell patients to report pregnancies to their physicians as soon as possible
[see Warnings and Precautions (
5.1) and Use in Specific Populations
(8.1)]
.
Lactation
Advise nursing women not to breastfeed during treatment with MICARDIS
[see Use in Specific Populations
(8.2)].
Symptomatic Hypotension and Syncope
Advise patients that lightheadedness can occur, especially during the first days of therapy, and to report it to their healthcare provider. Inform patients that inadequate fluid intake, excessive perspiration, diarrhea, or vomiting can lead to an excessive fall in blood pressure, with the same consequences of lightheadedness and possible syncope. Advise patients to contact their healthcare provider if syncope occurs
[see Warnings and Precautions
(5.2)].
Potassium Supplements
Advise patients not to use potassium supplements or salt substitutes that contain potassium without consulting the prescribing healthcare provider
[see Warnings and Precautions
(5.3)].
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
Licensed from:
Boehringer Ingelheim International GmbH, Ingelheim, Germany
Copyright © 2022 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED
MICARDIS ®is a registered trademark of and used under license from Boehringer Ingelheim International GmbH.
Boehringer Ingelheim Pharmaceuticals, Inc. either owns or uses the ONTARGET ®and TRANSCEND™ trademarks under license.
The other brands listed are trademarks of their respective owners and are not trademarks of Boehringer Ingelheim Pharmaceuticals, Inc.
MICARDIS
®(my-CAR-dis)
(telmisartan tablets)
Read this Patient Information before you start taking MICARDIS tablets and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment.
What is the most important information I should know about MICARDIS tablets?
MICARDIS can cause harm or death to an unborn baby. Talk to your doctor about other ways to lower your blood pressure if you plan to become pregnant. If you get pregnant while taking MICARDIS, tell your doctor right away.
MICARDIS is a prescription medicine used:
- to treat high blood pressure (hypertension)
- in certain high risk people aged 55 years and older to help lower their risk of having certain cardiovascular problems such as stroke, heart attack, or death
It is not known if MICARDIS is safe and effective in children.
You should not take MICARDIS tablets if you are allergic (hypersensitive) to the active ingredient (telmisartan) or any of the other ingredients listed at the end of this leaflet.
For patients with diabetes, if you are taking MICARDIS you should not take aliskiren.
What should I tell my doctor before taking MICARDIS tablets?
Before you take MICARDIS tablets, tell your doctor if you:
- are pregnant or are planning to become pregnant. See "What is the most important information I should know about MICARDIS tablets?"
- are breast-feeding or plan to breast-feed. It is not known if MICARDIS passes into your breast milk. You and your doctor should decide if you will take MICARDIS tablets or breast-feed. You should not do both. Talk with your doctor about the best way to feed your baby if you take MICARDIS tablets.
- have liver problems
- have kidney problems
- have heart problems
- have any other medical conditions
Tell your doctor about all the medicines you take,including prescription and non-prescription medicines, vitamins, and herbal supplements.
For patients with diabetes, if you are taking MICARDIS you should not take aliskiren.
MICARDIS may affect the way other medicines work, and other medicines may affect how MICARDIS works. Especially tell your doctor if you take:
- aliskiren
- digoxin (Lanoxin ®)
- lithium (Lithobid ®, lithium carbonate, lithium citrate)
- aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs)
- other medicines used to treat your high blood pressure or heart problem
- water pills (diuretic)
Know the medicines you take. Keep a list of them and show it to your doctor or pharmacist when you get a new medicine.
How should I take MICARDIS tablets?
- Take MICARDIS tablets exactly as your doctor tells you to take it.
- Your doctor will tell you how much MICARDIS to take and when to take it.
- Do not change your dose unless your doctor tells you to.
- Take MICARDIS one time each day at the same time.
- Take MICARDIS tablets with or without food.
- If you miss a dose, take it as soon as you remember. If it is close to your next dose, do not take the missed dose. Take the next dose at your regular time.
- If you take too much MICARDIS, call your doctor, or go to the nearest hospital emergency room right away.
- Read the "How to Open the Blister"at the end of this leaflet before you use MICARDIS. Talk with your doctor if you do not understand the instructions.
What are the possible side effects of MICARDIS tablets?
MICARDIS tablets may cause serious side effects, including:
- Injury or death to your unborn baby.See "What is the most important information I should know about MICARDIS tablets?"
-
Low blood pressure (hypotension)is most likely to happen if you also:
- take water pills (diuretics)
- are on a low-salt diet
- get dialysis treatments
- have heart problems
- get sick with vomiting or diarrhea
- If you feel faint or dizzy, lie down and call your doctor right away.
-
Kidney problems,which may get worse if you already have kidney disease. You may have changes in your kidney test results, and you may need a lower dose of MICARDIS tablets. Call your doctor if you get:
- swelling in your feet, ankles, or hands
- unexplained weight gain
- Call your doctor right away if you get any of the symptoms listed above.
- High potassium in the blood (hyperkalemia).Your doctor may check your potassium levels as needed.
Rare, serious allergic reactions may happen. Tell your doctor right away if you get any of these symptoms:
- swelling of the face, tongue, throat
- difficulty breathing
- skin rash
The most common side effects of MICARDIS tablets include:
- sinus pain and congestion (sinusitis)
- back pain
- diarrhea
These are not all the possible side effects with MICARDIS tablets. Tell your doctor if you have any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store MICARDIS tablets?
- Store MICARDIS tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not remove MICARDIS tablets from blisters until right before you take them.
Keep MICARDIS tablets and all medicines out of the reach of children.
General information about MICARDIS tablets
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use MICARDIS tablets for a condition for which it was not prescribed. Do not give MICARDIS tablets to other people, even if they have the same condition you have. It may harm them.
This Patient Information leaflet summarizes the most important information about MICARDIS tablets. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about MICARDIS tablets that is written for health professionals.
For current prescribing information, scan the code or call Boehringer Ingelheim Pharmaceuticals, Inc. at 1-800-542-6257.

What are the ingredients in MICARDIS tablets?
Inactive Ingredients:sodium hydroxide, meglumine, povidone, sorbitol, and magnesium stearate
What is High Blood Pressure (Hypertension)?
Blood pressure is the force in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too much. MICARDIS tablets can help your blood vessels relax so your blood pressure is lower. Medicines that lower your blood pressure lower your chance of having a stroke or heart attack.
High blood pressure makes the heart work harder to pump blood throughout the body and causes damage to the blood vessels. If high blood pressure is not treated, it can lead to stroke, heart attack, heart failure, kidney failure, and vision problems.
Patients older than 55 years of age who have been diagnosed with blood vessel disease in the heart, legs, or brain (coronary, peripheral, or cerebral vascular disease) or diabetes with end organ damage (for example: kidney, heart, and brain) are at higher risk of cardiovascular events (for example: death from cardiovascular causes, stroke, and/or heart attack).
1. Tear (You may also use scissors to tear the blister apart)
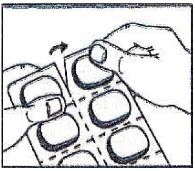
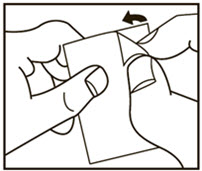
2. Peel (Peel off the paper layer from the aluminum foil)
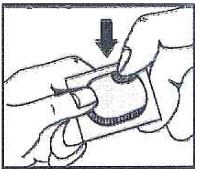
3. Push (Push the tablet through the aluminum foil)
This Patient Information has been approved by the U.S. Food and Drug Administration
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
Licensed from:
Boehringer Ingelheim International GmbH, Ingelheim, Germany
Copyright © 2022 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED
MICARDIS ®is a registered trademark of and used under license from Boehringer Ingelheim International GmbH.
Boehringer Ingelheim Pharmaceuticals, Inc. either owns or uses the ONTARGET ®and TRANSCEND™ trademarks under license.
The other brands listed are trademarks of their respective owners and are not trademarks of Boehringer Ingelheim Pharmaceuticals, Inc.
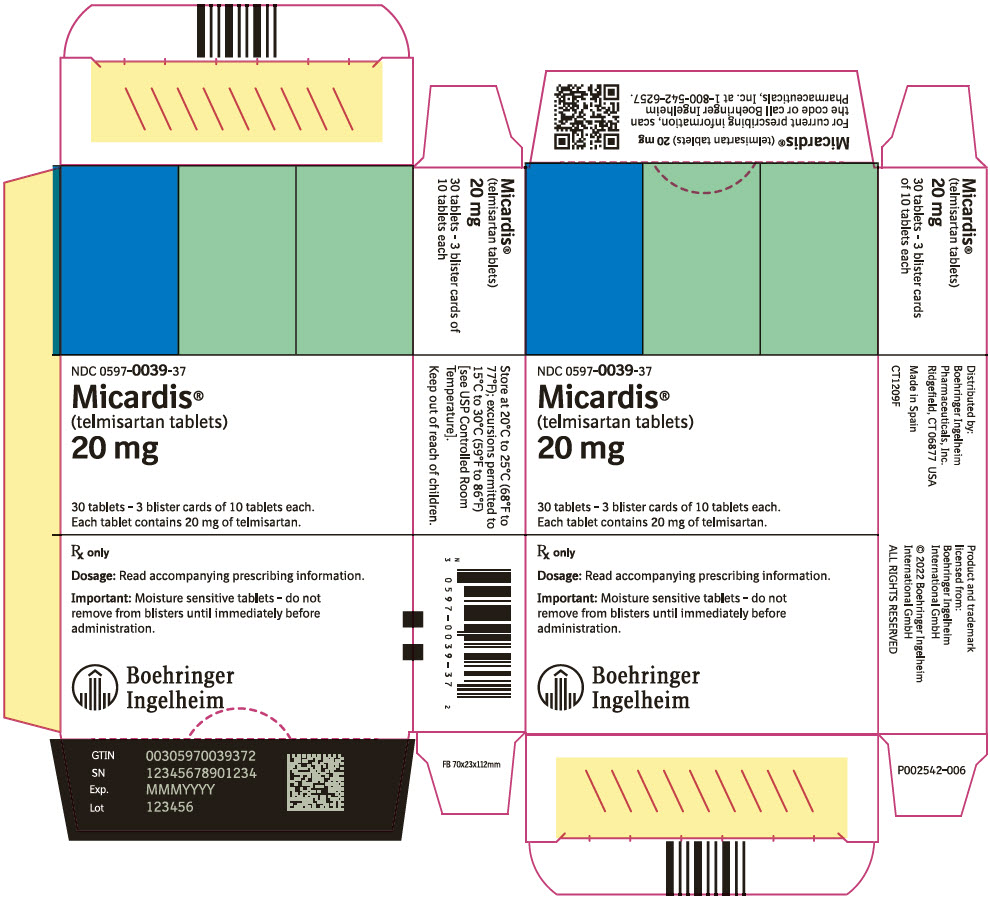
PRINCIPAL DISPLAY PANEL - 20 mg Tablet Blister Pack Carton
NDC: 0597-0039-37
Micardis®
(telmisartan tablets)
20 mg
30 tablets - 3 blister cards of 10 tablets each.
Each tablet contains 20 mg of telmisartan.
Rx only
Dosage: Read accompanying prescribing information.
Important: Moisture sensitive tablets - do not
remove from blisters until immediately before
administration.
Boehringer
Ingelheim
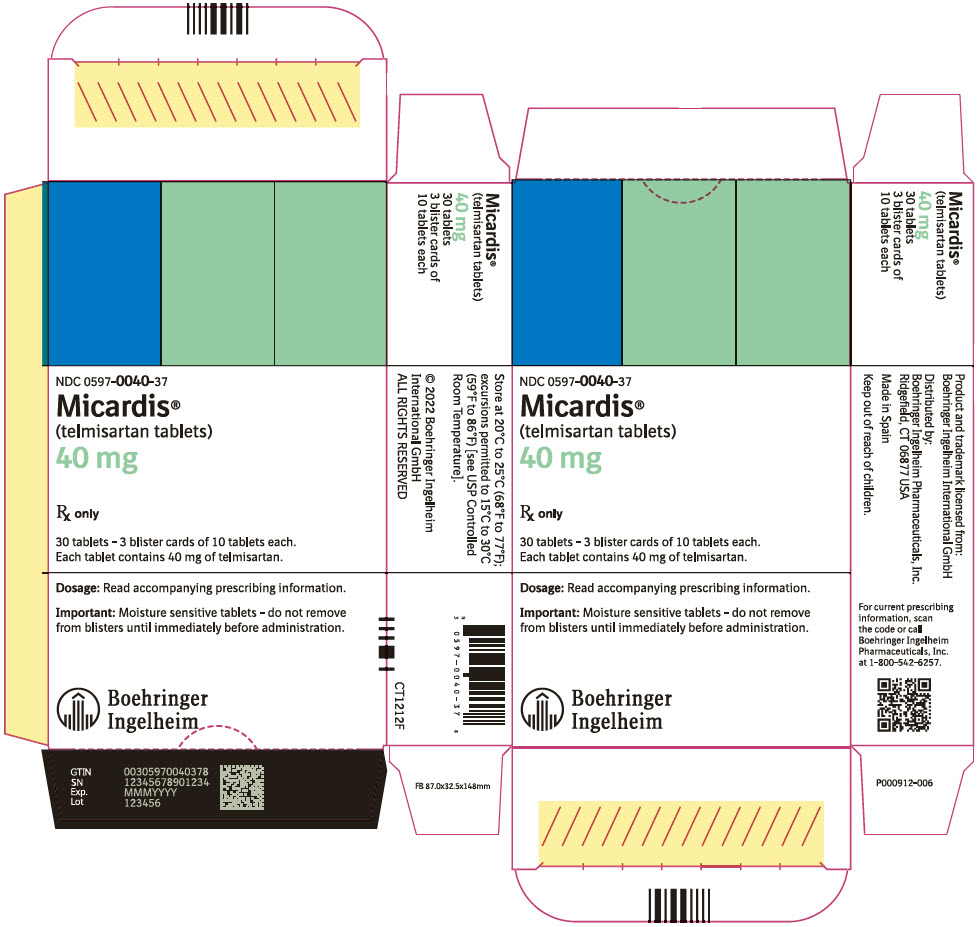
PRINCIPAL DISPLAY PANEL - 40 mg Tablet Blister Pack Carton
NDC: 0597-0040-37
Micardis®
(telmisartan tablets)
40 mg
Rx only
30 tablets - 3 blister cards of 10 tablets each.
Each tablet contains 40 mg of telmisartan.
Dosage: Read accompanying prescribing information.
Important: Moisture sensitive tablets - do not remove
from blisters until immediately before administration.
Boehringer
Ingelheim
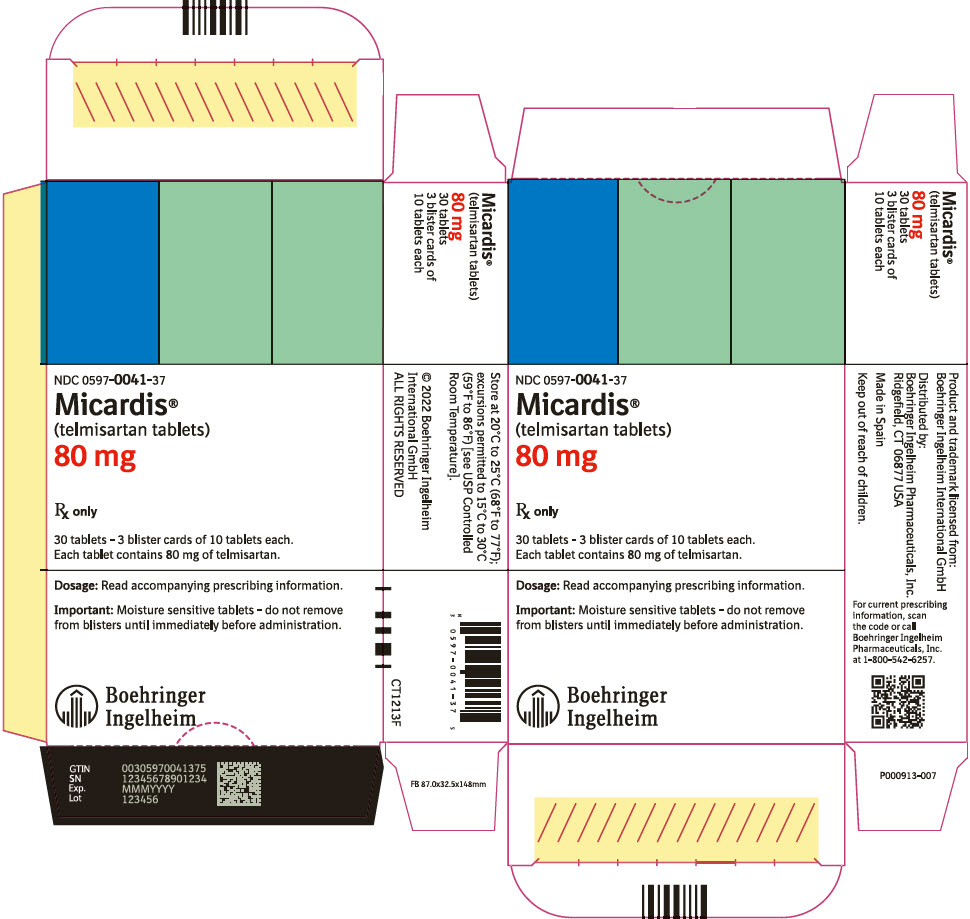
PRINCIPAL DISPLAY PANEL - 80 mg Tablet Blister Pack Carton
NDC: 0597-0041-37
Micardis®
(telmisartan tablets)
80 mg
Rx only
30 tablets - 3 blister cards of 10 tablets each.
Each tablet contains 80 mg of telmisartan.
Dosage: Read accompanying prescribing information.
Important: Moisture sensitive tablets - do not remove
from blisters until immediately before administration.
Boehringer
Ingelheim
| MICARDIS
telmisartan tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| MICARDIS
telmisartan tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| MICARDIS
telmisartan tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Praxis, LLC (016329513) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Praxis, LLC | 016329513 | manufacture(59368-382, 59368-383, 59368-384) , label(59368-382, 59368-383, 59368-384) , pack(59368-382, 59368-383, 59368-384) | |
Trademark Results [Micardis]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MICARDIS 75144120 2237153 Live/Registered |
BOEHRINGER INGELHEIM PHARMA GMBH & CO. KG 1996-08-02 |
 MICARDIS 73741159 1549781 Dead/Cancelled |
BOEHRINGER INGELHEIM KG 1988-07-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.