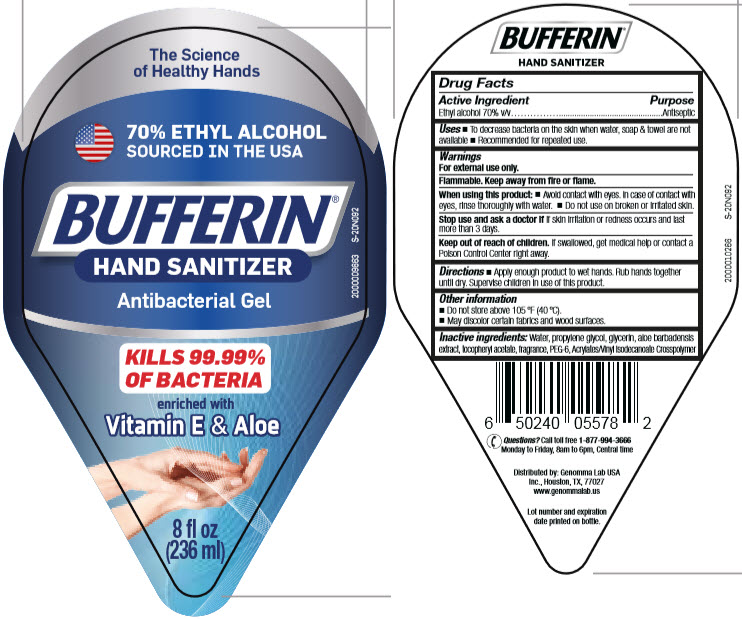
BUFFERIN® HAND SANITIZER
Bufferin Hand Sanitizer by
Drug Labeling and Warnings
Bufferin Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Genomma Lab USA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BUFFERIN HAND SANITIZER- alcohol gel
Genomma Lab USA
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
BUFFERIN® HAND SANITIZER
Uses
- To decrease bacteria on the skin when water, soap & towel are not available
- Recommended for repeated use.
Warnings
For external use only.
Flammable. Keep away from fire or flame.
Directions
- Apply enough product to wet hands. Rub hands together until dry. Supervise children in use of this product.
Other information
- Do not store above 105 °F (40 °C).
- May discolor certain fabrics and wood surfaces.
| BUFFERIN HAND SANITIZER
alcohol gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Genomma Lab USA (832323534) |
Revised: 7/2022
Document Id: 87918f3c-2a49-4d21-802f-287f8216eecb
Set id: 37060747-b875-4417-a895-4838176612be
Version: 3
Effective Time: 20220718
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.