POLYETHYLENE GLYCOL 3350 powder, for solution
Polyethylene Glycol 3350 by
Drug Labeling and Warnings
Polyethylene Glycol 3350 by is a Otc medication manufactured, distributed, or labeled by Kesin Pharma Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Polyethylene Glycol 3350 increases frequency of bowel movements and softens stools
- Active ingredient (in each dose)
- Purpose
- Use
-
Warnings
Ask a doctor before use if you have
- nausea, vomiting or abdominal pain
- a sudden change in bowel habits that lasts over 2 weeks
- irritable bowel syndrome
-
Directions
- do not take more than directed unless advised by your doctor
- adults and children 17 yearsof age and older:
- use once a day
- stir and dissolve one packet of powder (17 g) in any 4 to 8 ounce of beverage (cold, hot or room temperature) then drink
- do not combine with starch-based thickners used for difficulty swallowing
- ensure that the powder is fully dissolved before drinking
- do not drink if there are any clumps
- do not use more than 7 days
- children 16 years of age or under: ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 81033-762-14
Polyethylene Glycol 3350 Powder for Oral Solution
Osmotic Laxative
14 Packets
(14 once-daily-doses)

-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 81033-762-30
Polyethylene Glycol 3350 Powder for Oral Solution
Osmotic Laxative
30 Packets
(30 once-daily doses)

-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 81033-762-01
Polyethylene Glycol 3350 Powder for Oral Solution
Osmotic laxative
100 Packets
(100 once-daily doses)
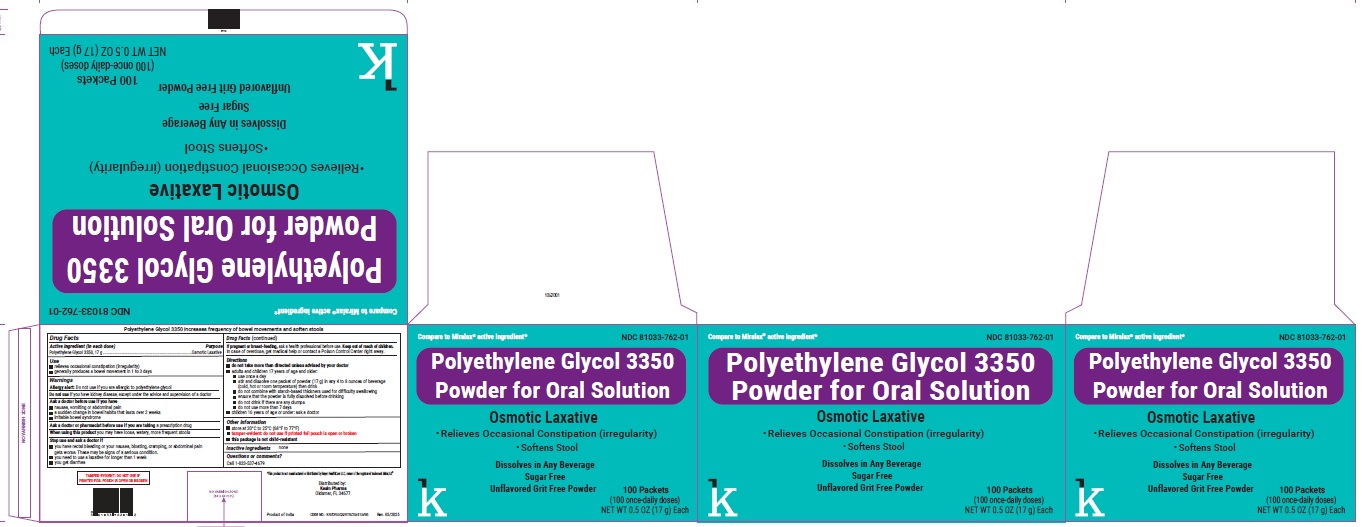
-
INGREDIENTS AND APPEARANCE
POLYETHYLENE GLYCOL 3350
polyethylene glycol 3350 powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 81033-762(NDC:59556-762) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 17 g in 17 g Product Characteristics Color white (Colorless upon dissolution) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 81033-762-14 14 in 1 CARTON 06/09/2025 1 NDC: 81033-762-17 17 g in 1 PACKET; Type 0: Not a Combination Product 2 NDC: 81033-762-30 30 in 1 CARTON 06/09/2025 2 NDC: 81033-762-17 17 g in 1 PACKET; Type 0: Not a Combination Product 3 NDC: 81033-762-01 100 in 1 CARTON 06/09/2025 3 NDC: 81033-762-17 17 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203928 06/09/2025 Labeler - Kesin Pharma Corporation (117447816) Establishment Name Address ID/FEI Business Operations Kesin Pharma Corporation 117447816 label(81033-762) , pack(81033-762) , repack(81033-762)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
