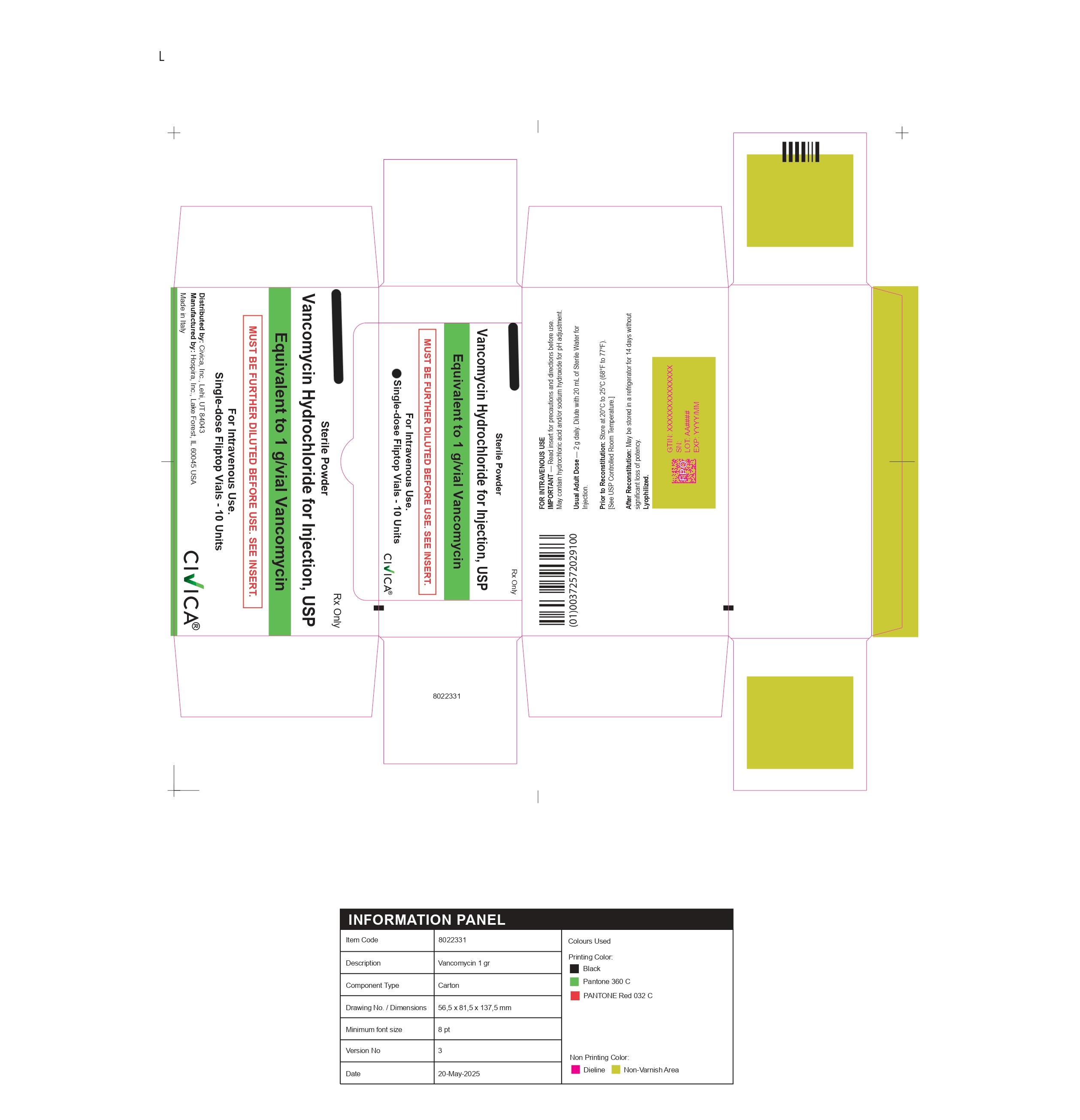
Vancomycin Hydrochloride by Avara Liscate Pharmaceuticals Services Spa
Vancomycin Hydrochloride by
Drug Labeling and Warnings
Vancomycin Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Avara Liscate Pharmaceuticals Services Spa. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VANCOMYCIN HYDROCHLORIDE- vancomycin hydrochloride injection, powder, lyophilized, for solution
Avara Liscate Pharmaceuticals Services Spa
----------
| VANCOMYCIN HYDROCHLORIDE
vancomycin hydrochloride injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Avara Liscate Pharmaceuticals Services Spa (564165541) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Avara Liscate Pharmaceuticals Services Spa | 564165541 | analysis(72132-0290) , manufacture(72132-0290) , label(72132-0290) , pack(72132-0290) | |
Revised: 10/2025
Document Id: 402ceb57-fdda-f25e-e063-6394a90ae91b
Set id: 3739c475-9f6f-bda2-e063-6294a90a5791
Version: 2
Effective Time: 20251002
Av
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.