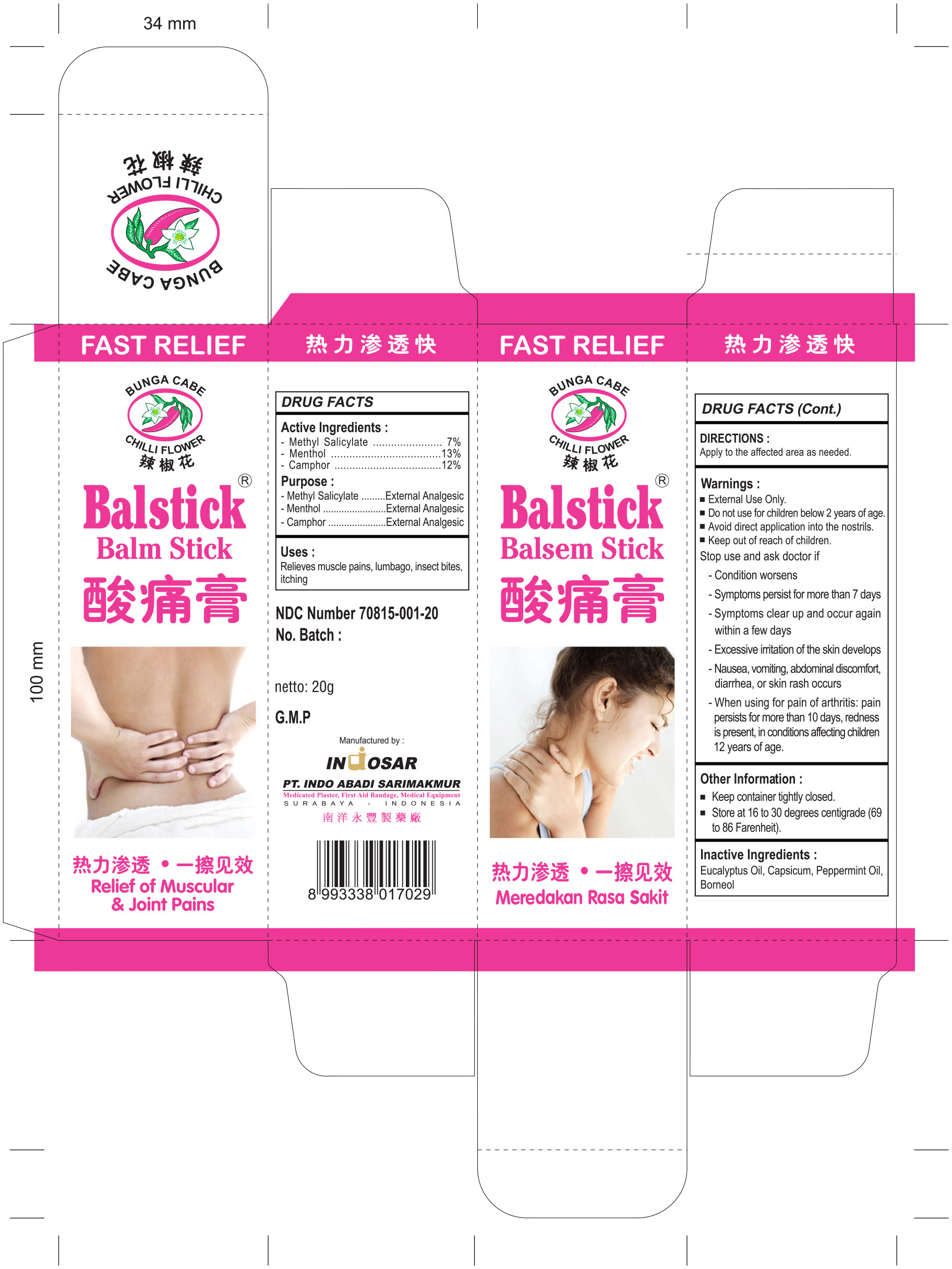
BALM STICK- camphor, menthol, methyl salicylate stick
Balm Stick by
Drug Labeling and Warnings
Balm Stick by is a Otc medication manufactured, distributed, or labeled by Indo Abadi Sari Makmur, PT. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients
- Purpose
- Uses
- DIRECTIONS
- Warnings
- Warnings
-
Warnings
Stop use and ask doctor if
Condition worsens
Symptoms persist for more than 7 days
Symptoms clear up and occur again within a few days
Excessive irritation of the skin develops
Nausea, vomitting, abdominal discomfort, diarrhea, or skin rash occurs
When using for pain of arthritis: pain persits for more than 10 days, redness is present, in conditions affecting children 12 years of age.
- Other Information
- Inactive Ingredients
- Drug Facts
-
INGREDIENTS AND APPEARANCE
BALM STICK
camphor, menthol, methyl salicylate stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70815-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 7 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 13 g in 100 g CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 12 g in 100 g Inactive Ingredients Ingredient Name Strength EUCALYPTUS OIL (UNII: 2R04ONI662) CAPSICUM (UNII: 00UK7646FG) PEPPERMINT OIL (UNII: AV092KU4JH) BORNEOL (UNII: M89NIB437X) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70815-001-20 1 in 1 CARTON 07/31/2016 1 20 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 07/31/2016 Labeler - Indo Abadi Sari Makmur, PT (728715202)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.