CROMOLYN SODIUM spray, metered
Cromolyn Sodium by
Drug Labeling and Warnings
Cromolyn Sodium by is a Otc medication manufactured, distributed, or labeled by Bausch & Lomb Incorporated, Bausch Health Companies, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (per spray)
- Purpose
- Uses
-
Warnings
When using this product
- it may take several days of use to notice an effect. Your best effect may not be seen for 1 to 2 weeks.
- brief stinging or sneezing may occur right after use
- do not use it to treat sinus infection, asthma, or cold symptoms
- do not share this bottle with anyone else as this may spread germs
-
Directions
- see package insert on how to use pump
- parent or care provider must supervise the use of this product by young children
-
adults and children 2 years and older:
- spray once into each nostril. Repeat 3-4 times a day (every 4-6 hours). If needed, may be used up to 6 times a day.
- use every day while in contact with the cause of your allergies (pollen, molds, pets, and dust)
- to prevent nasal allergy symptoms, use before contact with the cause of your allergies. For best results, start using up to one week before contact.
- if desired, you can use this product with other medicines, including other allergy medicines.
- Children under 2 years: Do not use unless directed by a doctor.
- Other information
- Inactive ingredients
- Questions?
-
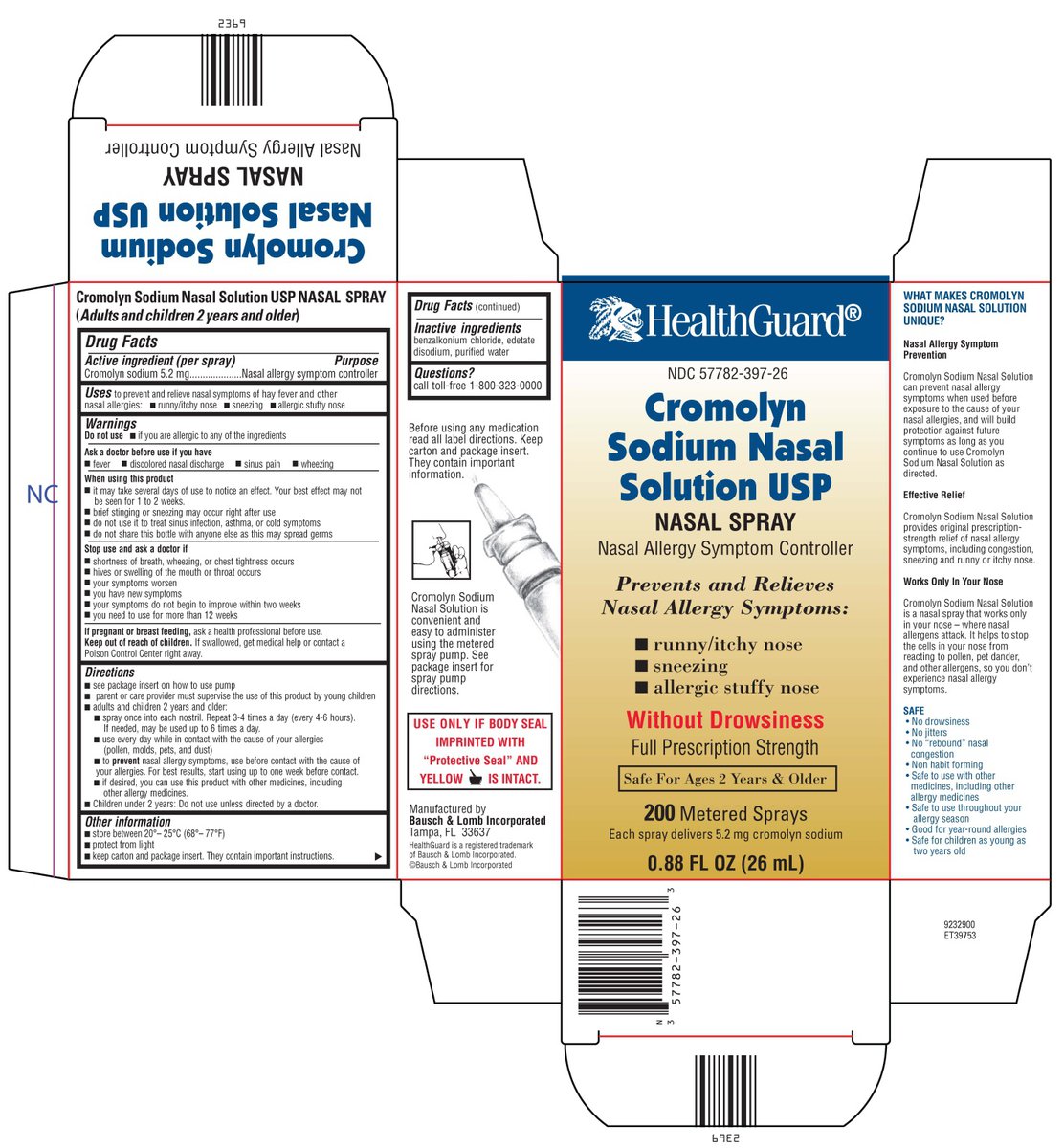
Package/Label Principal Display Panel
HealthGuard
NDC: 57782-397-26
Cromolyn Sodium Nasal Solution USP
NASAL SPRAY
Nasal Allergy Symptom Controller
Prevents and Relieves Nasal Allergy Symptoms:
runny/itchy nose
sneezing
allergic stuffy noseWithout Drowsiness
Full Prescription Strength
Safe For Ages 2 Years & Older
200 Metered Sprays
Each spray delivers 5.2 mg cromolyn sodium
0.88 FL OZ (26 mL)
-
INGREDIENTS AND APPEARANCE
CROMOLYN SODIUM
cromolyn sodium spray, meteredProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 57782-397 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CROMOLYN SODIUM (UNII: Q2WXR1I0PK) (CROMOLYN - UNII:Y0TK0FS77W) CROMOLYN SODIUM 5.2 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57782-397-26 1 in 1 CARTON 07/03/2001 1 26 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075702 07/03/2001 Labeler - Bausch & Lomb Incorporated (196603781) Establishment Name Address ID/FEI Business Operations Bausch & Lomb Incorporated 079587625 MANUFACTURE(57782-397) Establishment Name Address ID/FEI Business Operations Cambrex Profarmaco Milano Srl 438051401 API MANUFACTURE(57782-397) Establishment Name Address ID/FEI Business Operations Aventis Pharma Manufacturing Pte LTD. 595299306 API MANUFACTURE(57782-397)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.