FLUOCINONIDE by STAT RX USA LLC FLUOCINONIDE cream
FLUOCINONIDE by
Drug Labeling and Warnings
FLUOCINONIDE by is a Prescription medication manufactured, distributed, or labeled by STAT RX USA LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents. Fluocinonide Cream contains: Fluocinonide USP (Pregna-1,4-diene-3,20-dione, 21-(acetyloxy)-6,9-difluoro-11-hydroxy-16,17-[(1-methylethylidene)bis(oxy)]-, (6α,11β,16α)-). It has a molecular formula of C26H32F2O7 and a molecular weight of 494.53. It has the following structural formula:
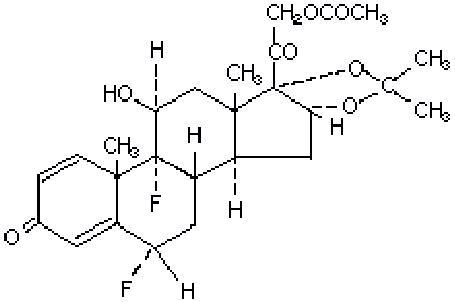
Each gram of the 0.05% cream contains: fluocinonide 0.5 mg in a cream base consisting of stearyl alcohol, propylene glycol, 1,2,6-hexanetriol, polyethylene glycol 8000, and citric acid. This white cream vehicle is greaseless, non-staining and completely water miscible. The base provides emollient and hydrophilic properties. In this formulation, the active ingredient is totally in solution.
-
CLINICAL PHARMACOLOGY
Topical corticosteroids share anti-inflammatory, antipruritic and vasoconstrictive actions.
The mechanism of anti-inflammatory activity of the topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies and/or clinical efficacies of the topical corticosteroids. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
Pharmacokinetics: The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase the percutaneous absorption. Occlusive dressings substantially increase the percutaneous absorption of topical corticosteroids. Thus, occlusive dressings may be a valuable therapeutic adjunct for treatment of resistant dermatoses (See DOSAGE AND ADMINISTRATION).
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Corticosteroids are bound to plasma proteins in varying degrees. Corticosteroids are metabolized primarily in the liver and are then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
General: Systemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing's syndrome, hyperglycemia, and glucosuria in some patients.
Conditions which augment systemic absorption include the application of the more potent steroids, use over large surface areas, prolonged use, and the addition of occlusive dressings. Therefore, patients receiving a large dose of a potent topical steroid applied to a large surface area or under an occlusive dressing should be evaluated periodically for evidence of HPA axis suppression by using the urinary free cortisol and ACTH stimulation tests. If HPA axis suppression is noted, an attempt should be made to withdraw the drug, to reduce the frequency of application, or to substitute a less potent steroid.
Recovery of HPA axis function is generally prompt and complete upon discontinuation of the drug. Infrequently, signs and symptoms of steroid withdrawal may occur, requiring the use of supplemental systemic corticosteroids.
Children may absorb proportionally larger amounts of topical corticosteroids and thus be more susceptible to systemic toxicity (See PRECAUTIONS - Pediatric Use). If irritation develops, topical corticosteroids should be discontinued and appropriate therapy instituted.
As with any topical corticosteroid product, prolonged use may produce atrophy of the skin and subcutaneous tissues. When used on intertriginous or flexor areas, or on the face, this may occur even with short-term use.
In the presence of dermatological infections, the use of an appropriate antifungal or antibacterial agent should be instituted. If a favorable response does not occur promptly, the corticosteroid should be discontinued until the infection has been adequately controlled.
Information for Patients: Patients using topical corticosteroids should receive the following information and instructions:
- This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes.
- Patients should be advised not to use this medication for any disorder other than for which it was prescribed.
- The treated skin area should not be bandaged or otherwise covered or wrapped as to be occlusive unless directed by the physician.
- Patients should report any signs of local adverse reactions especially under occlusive dressings.
- Parents of pediatric patients should be advised not to use tight fitting diapers or plastic pants on a child being treated in the diaper area, as these garments may constitute occlusive dressings.
Laboratory Tests: The following tests may be helpful in evaluating the HPA axis suppression: Urinary free cortisol test; ACTH stimulation test.
Carcinogenesis, Mutagenesis, and Impairment of Fertility: Long-term animal studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of topical corticosteroids. Studies to determine mutagenicity with prednisolone and hydrocortisone have revealed negative results.
Pregnancy:Teratogenic Effects: Pregnancy Category C: Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively low dosage levels. The more potent corticosteroids have been shown to be teratogenic after dermal application in laboratory animals. There are no adequate and well-controlled studies in pregnant women on teratogenic effects from topically applied corticosteroids. Therefore, topical corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Drugs of this class should not be used extensively on pregnant patients, in large amounts, or for prolonged periods of time.
Nursing Mothers: It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Systemically administered corticosteroids are secreted into breast milk in quantities not likely to have a deleterious effect on the infant. Nevertheless, caution should be exercised when topical corticosteroids are administered to a nursing woman.
Pediatric Use:Pediatric patients may demonstrate greater susceptibility to topical corticosteroid-induced HPA axis suppression and Cushing's syndrome than mature patients because of a larger skin surface area to body weight ratio.
Hypothalamic-pituitary-adrenal (HPA) axis suppression, Cushing's syndrome, and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include linear growth retardation, delayed weight gain, low plasma cortisol levels, and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
Administration of topical corticosteroids to children should be limited to the least amount compatible with an effective therapeutic regimen. Chronic corticosteroid therapy may interfere with the growth and development of children.
-
ADVERSE REACTIONS
The following local adverse reactions are reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings. These reactions are listed in an approximate decreasing order of occurrence: burning, itching, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infection, skin atrophy, striae and miliaria.
-
OVERDOSAGE
Topically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects (See PRECAUTIONS).
-
DOSAGE AND ADMINISTRATION
Fluocinonide Cream 0.05% is generally applied to the affected area as a thin film from two to four times daily depending on the severity of the condition.
Occlusive dressings may be used for the management of psoriasis or recalcitrant conditions. If an infection develops, the use of occlusive dressings should be discontinued and appropriate antimicrobial therapy instituted.
-
HOW SUPPLIED
Fluocinonide Cream USP, 0.05% in:
- 15 gram tubes, NDC: 0168-0139-15
- 30 gram tubes, NDC: 0168-0139-30
- 60 gram tubes, NDC: 0168-0139-60
Store at controlled room temperature 15°-30°C (59°-86°F).
Avoid excessive heat above 40°C (104°F).
E. FOUGERA & CO.
a division of Nycomed US Inc.
Melville, New York 11747 USAI21915B
R2/08
#54 -
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 60 G CARTON
NDC: 0168-0139-15
FOUGERA® Rx only
FLUOCINONIDE
CREAM USP, 0.05%
WARNING: Keep out of
reach of children.FOR EXTERNAL USE ONLY
NOT FOR OPHTHALMIC USE
KEEP CONTAINER TIGHTLY CLOSED.
NET WT 60 grams

-
INGREDIENTS AND APPEARANCE
FLUOCINONIDE
fluocinonide creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 16590-368(NDC:0168-0139) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUOCINONIDE (UNII: 2W4A77YPAN) (FLUOCINONIDE - UNII:2W4A77YPAN) FLUOCINONIDE 0.5 mg in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 16590-368-60 60 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA073030 10/17/1994 Labeler - STAT RX USA LLC (786036330)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.