ZONISADE- zonisamide suspension
ZONISADE by
Drug Labeling and Warnings
ZONISADE by is a Prescription medication manufactured, distributed, or labeled by Praxis, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiepileptic drugs, such as ZONISADE, during pregnancy. To provide information regarding the effects of in utero exposure to ZONISADE, physicians are advised to recommend that pregnant patients taking ZONISADE enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. This can be done by calling the toll-free number 1-888-233-2334 and must be done by patients themselves. Information on the registry can also be found at the website http://www.aedpregnancyregistry.org/.
Risk Summary
Based on findings from animal studies, ZONISADE may cause fetal harm when administered to a pregnant woman. Zonisamide causes metabolic acidosis in humans [see Warnings and Precautions ( 5.8)] . There are no reports of metabolic acidosis with use of zonisamide in pregnancy; however, there are published prospective cohort studies that suggest an increased rate of small for gestational age infants in pregnancies exposed to zonisamide, which may be associated with metabolic acidosis (see Clinical Considerations and Data).
The available published data from the NAAED Pregnancy Registry has not identified a drug-associated risk of major birth defects with zonisamide use in pregnancy. Although a small prospective cohort study reported an increased risk of major birth defects in zonisamide-exposed pregnancies, this study has methodologic limitations, including small sample size and inability to account for potential confounders (see Data).The available published data pertaining to the use of zonisamide during pregnancy are insufficient to evaluate for a drug-associated risk of miscarriage.
In animal studies, administration of zonisamide during pregnancy produced fetal malformations in multiple species and embryofetal (monkey) or perinatal (rat) death at maternal plasma levels similar to or lower than therapeutic levels in humans [see Warnings and Precautions ( 5.10) and Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the general U.S. population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Dose Adjustments During Pregnancy and the Postpartum Period
As with other AEDs, physiological changes during pregnancy may affect zonisamide concentrations and/or therapeutic effect. There have been reports of decreased zonisamide concentrations during pregnancy and restoration of pre-pregnancy concentrations after delivery. Dose adjustments may be necessary to maintain clinical response.
Maternal Adverse Reactions
Metabolic acidosis in pregnancy (due to other causes) may be associated with decreased fetal growth, decreased fetal oxygenation, and fetal death, and may affect the fetus' ability to tolerate labor. There are no reports of metabolic acidosis or fetal death with use of zonisamide in pregnancy [see Warnings and Precautions ( 5.8)] .
Fetal/Neonatal Adverse Reactions
Newborns of mothers treated with zonisamide should be monitored for metabolic acidosis because of transfer of zonisamide to the fetus and possible occurrence of transient metabolic acidosis following birth. Transient metabolic acidosis has been reported in neonates born to mothers treated during pregnancy with a different carbonic anhydrase inhibitor.
Data
Human Data
A prospective cohort study from the NAAED Pregnancy Registry has not identified an increase in the rate of major birth defects (1.4%) in over 200 first trimester pregnancies exposed to zonisamide monotherapy use. Methodological limitations include small sample size and selection bias.
A prospective cohort study from the United Kingdom and Ireland Epilepsy Pregnancy Registry (UKIEPR) reported an increased rate of major birth defects (13%) in 26 first trimester pregnancies exposed to zonisamide monotherapy use. Methodological limitations include small sample size and inability to account for potential confounders.
Prospective cohort studies, including data from NAAED Pregnancy Registry and UKIEPR, have reported increased rates of small for gestational age infants in those exposed to zonisamide during pregnancy compared to lamotrigine-exposed pregnancies and the unexposed general population.
Animal Data
In mice, treatment of pregnant animals with zonisamide (0, 125, 250, or 500 mg/kg/day) during the period of organogenesis resulted in increased incidences of fetal malformations (skeletal and/or craniofacial defects) at all doses tested. A no-effect dose for adverse effects on embryofetal development in mice was not identified. The lowest dose tested was approximately 1.5 times that in humans at the maximum recommended human dose (MRHD) of 400 mg/day on a mg/m 2basis.
In rats, an increased frequency of malformations (cardiovascular defects) and variations (persistent cords of thymic tissue, decreased skeletal ossification) was observed in the offspring of dams treated with zonisamide (0, 20, 60, or 200 mg/kg/day) throughout organogenesis at all doses. A no-effect dose for adverse effects on embryofetal development in rats was not identified. The lowest dose tested was approximately 0.5 times the MRHD on a mg/m 2basis.
Following administration of zonisamide (0, 10, 30, or 60 mg/kg/day) to pregnant dogs during organogenesis, increased incidences of fetal cardiovascular malformations (ventricular septal defects, cardiomegaly, various valvular and arterial anomalies) were found at doses of 30 mg/kg/day or greater. Cardiovascular malformations were found in approximately 50% of all fetuses exposed to the high dose. Incidences of skeletal malformations were also increased at the high dose, and fetal growth retardation and increased frequencies of skeletal variations were seen at all doses. Plasma levels in pregnant dogs (12 μg/mL) at the low and mid doses tested (10 and 30 mg/kg, respectively) were lower than those in humans at the MRHD; plasma levels at the high dose tested in pregnant dogs were similar to those in humans at the MRHD.
In cynomolgus monkeys, administration of zonisamide (0, 10 or 20 mg/kg/day) to pregnant animals during organogenesis resulted in embryofetal deaths at both doses. The possibility that these deaths were due to malformations cannot be ruled out. A no-effect dose for embryofetal death was not identified. At the low dose tested, peak plasma levels in pregnant monkey were substantially lower than that in humans at the MRHD.
Perinatal death was increased among the offspring of rats treated with zonisamide (0, 10, 30, or 60mg/kg/day) from the latter part of gestation up to weaning at the high dose. The no-effect dose (30 mg/kg/day) for adverse peri- and postnatal developmental effects in rats is less than the MRHD on a body surface area (mg/m 2) basis.
8.2 Lactation
Risk Summary
Zonisamide is readily transferred to human milk, with a reported milk-to-plasma ratio ranging between 0.7 to 0.9 in the published lactation studies. There are no published reports of adverse effects on the breastfed infant exposed to zonisamide during breastfeeding. There are no data on the effect of zonisamide on milk production. Because ZONISADE has been associated with metabolic acidosis in adult and pediatric patients and hyperthermia in pediatric patients, infants exposed to ZONISADE during breastfeeding should be monitored for poor feeding, weight loss, excess sedation, decreased muscle tone, and elevated temperature [see Warnings and Precautions ( 5.8)] .
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ZONISADE and any potential adverse effects on the breastfed infant from ZONISADE or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Contraception
Females
Based on animal data, zonisamide can cause fetal harm when administered to a pregnant woman [see Warnings and Precautions ( 5.10)] . Advise females of reproductive potential to use effective contraception during treatment with ZONISADE and for one month after discontinuation.
Infertility
Females
Based on findings from animal fertility studies, ZONISADE may impair fertility in females [see Nonclinical Toxicology ( 13.1)] .
8.4 Pediatric Use
Safety and effectiveness of ZONISADE have been established in patients 16 years of age and older by evidence from adequate and well-controlled studies of zonisamide [see Clinical Studies ( 14)] .
Safety and effectiveness in pediatric patients below the age of 16 have not been established. Acute myopia and secondary angle closure glaucoma have been reported in pediatric patients [see Warnings and Precautions ( 5.6)] . Cases of oligohidrosis and hyperpyrexia have been reported [see Warnings and Precautions ( 5.5)] . Zonisamide commonly causes metabolic acidosis in pediatric patients [see Warnings and Precautions ( 5.8)] . Chronic untreated metabolic acidosis in pediatric patients may cause nephrolithiasis and/or nephrocalcinosis, osteoporosis and/or osteomalacia (potentially resulting in rickets), and may reduce growth rates. A reduction in growth rate may eventually decrease the maximal height achieved. The effect of zonisamide on growth and bone-related sequelae has not been systematically investigated.
8.5 Geriatric Use
Single dose pharmacokinetic parameters are similar in elderly and young healthy volunteers [see Clinical Pharmacology ( 12.3)] . Clinical studies of zonisamide did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Renal Impairment
ZONISADE is cleared via renal pathway [see Clinical Pharmacology ( 12.3)] . Patients with renal impairment might require slower titration, and more frequent monitoring is required. Avoid use of ZONISADE in patients with renal failure (estimated GFR < 50 mL/min). ZONISADE should be discontinued in patients who develop acute renal failure or a clinically significant sustained increase in the creatinine/BUN concentration [see Warnings and Precautions ( 5.14)].
-
10 OVERDOSAGE
10.1 Human Experience
During zonisamide clinical development, three patients ingested unknown amounts of zonisamide as suicide attempts, and all three were hospitalized with CNS symptoms. One patient became comatose and developed bradycardia, hypotension, and respiratory depression; the zonisamide plasma level was 100.1 μg/mL measured 31 hours post-ingestion. Zonisamide plasma levels fell with a half-life of 57 hours, and the patient became alert five days later.
10.2 Management
No specific antidotes for zonisamide overdosage are available. Following a suspected recent overdose, emesis should be induced or gastric lavage performed with the usual precautions to protect the airway. General supportive care is indicated, including frequent monitoring of vital signs and close observation.
Zonisamide has a long half-life [see Clinical Pharmacology ( 12.3)]. Due to the low protein binding of zonisamide (40%), renal dialysis may be effective. The effectiveness of renal dialysis as a treatment of overdose has not been formally studied. A poison control center should be contacted for information on the management of ZONISADE overdosage.
-
11 DESCRIPTION
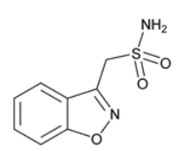
ZONISADE (zonisamide oral suspension) is chemically classified as a sulfonamide. The active ingredient is zonisamide, 1,2-benzisoxazole-3-methanesulfonamide. The empirical formula is C 8H 8N 2O 3S with a molecular weight of 212.23. Zonisamide is a white powder, pKa = 10.2, and is moderately soluble in water (0.80 mg/mL) and 0.1 N HCl (0.50 mg/mL).
The chemical structure is:
ZONISADE is an aqueous white to off-white liquid oral suspension. Each mL contains 20 mg of zonisamide. Inactive ingredients include carboxymethylcellulose sodium, citric acid monohydrate, microcrystalline cellulose, purified water, sodium benzoate, strawberry flavor, sucralose, trisodium citrate dihydrate, and xanthan gum.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The precise mechanism(s) by which zonisamide exerts its anticonvulsant effects is unknown. Zonisamide may produce these effects through action at sodium and calcium channels. In vitro pharmacological studies suggest that zonisamide blocks sodium channels and reduces voltage-dependent, transient inward currents (T-type Ca2+ currents), consequently stabilizing neuronal membranes. Other in vitro studies have demonstrated that zonisamide (10–30 µg/mL) suppresses synaptically-driven electrical activity without affecting postsynaptic GABA or glutamate responses (cultured mouse spinal cord neurons) or neuronal or glial uptake of [ 3H]-GABA (rat hippocampal slices). Thus, zonisamide does not appear to potentiate the synaptic activity of GABA. Zonisamide is a carbonic anhydrase inhibitor. The contribution of this pharmacological action to the therapeutic effects of zonisamide is unknown.
12.2 Pharmacodynamics
As a carbonic anhydrase inhibitor, ZONISADE may cause metabolic acidosis and may also increase the risks of hyperammonemia and kidney stone formation [see Warnings and Precautions ( 5.8, 5.13, 5.15) and Drug Interactions ( 7.2)].
12.3 Pharmacokinetics
Absorption
Following a 100 mg ZONISADE dose in normal volunteers, the time to maximum plasma concentrations (T max) occurred within 0.5–5 hours.
Zonisamide pharmacokinetics are dose-proportional in the range of 200 to 400 mg. Once a stable dose is reached, steady state is achieved within 14 days.
Effect of Food
When ZONISADE is administered with food, the zonisamide T maxis delayed, occurring at 3.5–7.5 hours, but food has no effect on the bioavailability of zonisamide.
Distribution
The apparent volume of distribution (V/F) of zonisamide is about 1.45 L/kg following a 400 mg oral dose. Zonisamide, at concentrations of 1.0–7.0 mcg/mL, is approximately 40% bound to human plasma proteins. Zonisamide extensively binds to erythrocytes, resulting in an eight-fold higher concentration of zonisamide in red blood cells than in plasma. Protein binding of zonisamide is unaffected in the presence of therapeutic concentrations of phenytoin, phenobarbital, or carbamazepine.
Elimination
The plasma clearance of oral zonisamide is approximately 0.30–0.35 mL/min/kg in patients not receiving enzyme-inducing antiepileptic drugs (AEDs). The clearance of zonisamide is increased to 0.5 mL/min/kg in patients concurrently on enzyme-inducing AEDs (see Potential for Other Drugs to Affect ZONISADE). After a single-dose administration, renal clearance of zonisamide is approximately 3.5 mL/min.
Metabolism
Zonisamide is metabolized by N-acetyl-transferases to form N-acetyl zonisamide and by CYP3A4 to form 2–sulfamoylacetylphenol (SMAP).
Excretion
The elimination half-life of zonisamide in plasma is approximately 63 hours. The elimination half-life of zonisamide in red blood cells is approximately 105 hours. Zonisamide is excreted primarily in urine as parent drug and as the glucuronide of a metabolite. Following multiple dosing, 62% of the radiolabeled dose was recovered in the urine, with 3% in the feces by day 10. Of the excreted dose, 35% was recovered as zonisamide, 15% as N-acetyl zonisamide, and 50% as the glucuronide of SMAP.
Specific Populations
Patients with Renal Impairment
Single 300 mg zonisamide doses were administered to three groups of volunteers. Group 1 was a healthy group with a creatinine clearance ranging from 70–152 mL/min. Group 2 and Group 3 had creatinine clearances ranging from 14.5–59 mL/min and 10–20 mL/min, respectively. Zonisamide renal clearance decreased with decreasing renal function (3.42, 2.50, and 2.23 mL/min, respectively). Marked renal impairment (creatinine clearance < 20 mL/min) was associated with an increase in zonisamide AUC of 35% [see Use in Specific Populations ( 8.6)] .
Patients with Hepatic Impairment
The pharmacokinetics of zonisamide in patients with impaired liver function have not been studied .
Age
The pharmacokinetics of a 300 mg single dose of zonisamide were similar in young (mean age 28 years) and elderly subjects (mean age 69 years).
Drug Interaction Studies
In-Vitro Studies
Enzymes
In vitro studies using human liver microsomes show insignificant (<25%) inhibition of cytochrome P450 isozymes 1A2, 2A6, 2C9, 2C19, 2D6, 2E1, 3A4, 2B6, or 2C8 at zonisamide levels approximately two-fold or greater than clinically relevant unbound serum concentrations. Therefore, ZONISADE is not expected to affect the pharmacokinetics of other drugs via cytochrome P450-mediated mechanisms.
Transporters
An in-vitrostudy showed that zonisamide is a weak inhibitor of P-gp (MDR1).
In-Vivo Studies
Potential for Zonisamide to Affect Other Drugs
Antiepileptic Drugs
In epileptic patients, steady state dosing with zonisamide capsules resulted in no clinically relevant pharmacokinetic effects on carbamazepine, lamotrigine, phenytoin, or sodium valproate.
Oral Contraceptives
In healthy subjects, steady state dosing with zonisamide capsules did not affect serum concentrations of ethinylestradiol or norethisterone in a combined oral contraceptive.
CYP2D6 Substrates
Coadministration of multiple dosing of zonisamide up to 400 mg/day with single 50-mg doses of desipramine did not significantly affect the pharmacokinetic parameters of desipramine, a probe drug for CYP2D6 activity.
Potential for Other Drugs to Affect ZONISADE
CYP3A4 Inducers
The half-life of zonisamide following a 400 mg dose in patients concurrently on enzyme-inducing AEDs such as phenytoin, carbamazepine, or phenobarbital, was between 27-38 hours; the half-life of zonisamide in patients concurrently on the non-enzyme inducing AED, valproate, was 46 hours.
These effects are unlikely to be of clinical significance when ZONISADE is added to existing therapy; however, changes in zonisamide concentrations may occur if concomitant CYP3A4 inducing antiepileptic or other drugs are withdrawn, dose adjusted or introduced, an adjustment of the ZONISADE dose may be required [see Drug Interactions ( 7.3)] .
CYP3A4 Inhibitors
Steady-state dosing of either ketoconazole (400 mg/day) or cimetidine (1200 mg/day) had no clinically relevant effects on the single dose pharmacokinetics of zonisamide given to healthy subjects.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenicity, Mutagenesis, Impairment of Fertility
Carcinogenicity
No evidence of carcinogenicity was found in mice or rats following dietary administration of zonisamide for two years at doses of up to 80 mg/kg/day. In mice, this dose is approximately equivalent to the maximum recommended human dose (MRHD) of 400 mg/day on a mg/m2 basis. In rats, this dose is 1–2 times the MRHD on a mg/m2 basis.
Mutagenesis
Zonisamide was mutagenic in an in vitro chromosomal aberration assay in CHL cells. Zonisamide was not mutagenic or clastogenic in other in vitro assays (Ames, mouse lymphoma tk assay, chromosomal aberration in human lymphocytes) or in the in vivo rat bone marrow cytogenetics assay.
Impairment of Fertility
Rats treated with zonisamide (20, 60, or 200 mg/kg) before mating and during the initial gestation phase showed signs of reproductive toxicity (decreased corpora lutea, implantations, and live fetuses) at all doses. The low dose in this study is approximately 0.5 times the maximum recommended human dose (MRHD) on a mg/m 2basis.
-
14 CLINICAL STUDIES
The efficacy of ZONISADE is based upon a bioavailability study comparing ZONISADE oral suspension to zonisamide capsules in healthy subjects. The clinical studies information described below pertains to the zonisamide capsule formulation.
The effectiveness of zonisamide as adjunctive therapy has been established in three multicenter, placebo-controlled, double blind, 3-month clinical trials (two domestic, one European) in 499 patients with refractory partial-onset seizures with or without secondary generalization. Each patient had a history of at least four partial-onset seizures per month in spite of receiving one or two antiepilepsy drugs at therapeutic concentrations. The 499 patients (209 women, 290 men) had a mean age of about 35 years. In the two US studies, over 80% of patients were Caucasian; 100% of patients in the European study were Caucasian. Zonisamide capsules or placebo was added to the existing therapy. The primary measure of effectiveness was median percent reduction from baseline in partial seizure frequency. The secondary measure was proportion of patients achieving a 50% or greater seizure reduction from baseline (responders). The results described below are for all partial seizures in the intent-to-treat populations.
In the first study (n = 203), all patients had a 1-month baseline observation period, then received placebo or zonisamide capsules in one of two dose escalation regimens; either 1) 100 mg/day for five weeks, 200 mg/day for one week, 300 mg/day for one week, and then 400 mg/day for five weeks; or 2) 100 mg/day for one week, followed by 200 mg/day for five weeks, then 300 mg/day for one week, then 400 mg/day for five weeks. This design allowed a 100 mg vs. placebo comparison over weeks 1–5, and a 200 mg vs. placebo comparison over weeks 2–6; the primary comparison was 400 mg (both escalation groups combined) vs. placebo over weeks 8–12. The total daily dose was given as twice a day dosing. Statistically significant treatment differences favoring zonisamide were seen for doses of 100, 200, and 400 mg/day.
In the second (n = 152) and third (n = 138) studies, patients had a 2–3 month baseline, then were randomly assigned to placebo or zonisamide capsules for three months. Zonisamide was introduced by administering 100 mg/day for the first week, 200 mg/day the second week, then 400 mg/day for two weeks, after which the dose could be adjusted as necessary to a maximum dose of 20 mg/kg/day or a maximum plasma level of 40 µg/mL. In the second study, the total daily dose was given as twice a day dosing; in the third study, it was given as a single daily dose. The average final maintenance doses received in the studies were 530 and 430 mg/day in the second and third studies, respectively. Both studies demonstrated statistically significant differences favoring zonisamide for doses of 400–600 mg/day, and there was no apparent difference between once daily and twice daily dosing (in different studies). Analysis of the data (first 4 weeks) during titration demonstrated statistically significant differences favoring zonisamide at doses between 100 and 400 mg/day. The primary comparison in both trials was for any dose over Weeks 5–12.
Table 3. Median % Reduction in All Partial-Onset Seizures and % Responders in Primary Efficacy Analyses: Intent-To-Treat Analysis - * p<0.05 compared to placebo
Study
Median % Reduction
in Partial-Onset Seizures% Responders
Zonisamide Capsules
Placebo
Zonisamide Capsules
Placebo
Study 1:
n=98
n=72
n=98
n=72
Weeks 8-12:
40.5% *
9.0%
41.8% *
22.2%
Study 2:
n=69
n=72
n=69
n=72
Weeks 5-12:
29.6% *
-3.2%
29.0%
15.0%
Study 3:
n=67
n=66
n=67
n=66
Weeks 5-12:
27.2% *
-1.1%
28.0% *
12.0%
Table 4. Median % Reduction in All Partial-Onset Seizures and % Responders for Dose Analyses in Study 1: Intent-To-Treat Analysis - * p<0.05 compared to placebo
Dose Group
Median % Reduction
in Partial-Onset Seizures% Responders
Zonisamide Capsules
Placebo
Zonisamide Capsules
Placebo
100-400 mg/day:
n=112
n=83
n=112
n=83
Weeks 1-12:
32.3% *
5.6%
32.1% *
9.6%
100 mg/day:
n=56
n=80
n=56
n=80
Weeks 1-5:
24.7% *
8.3%
25.0% *
11.3%
200 mg/day:
n=55
n=82
n=55
n=82
Weeks 2-6:
20.4% *
4.0%
25.5% *
9.8%
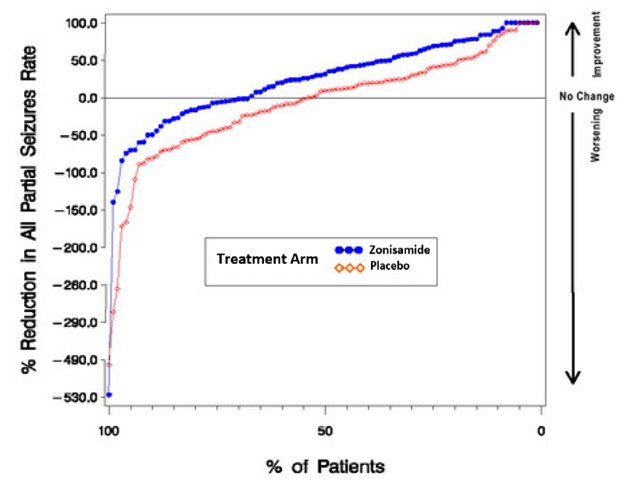
In Figure 1, a positive value on the Y-axis indicates an improvement from baseline (i.e., a decrease in seizure rate), while a negative value indicates a worsening from baseline (i.e., an increase in seizure rate). Thus, in a display of this type, the curve for an effective treatment is shifted to the left of the curve for placebo. The proportion of patients achieving any particular level of reduction in seizure rate was consistently higher for the zonisamide groups compared to the placebo groups. For example, Figure 1 indicates that approximately 27% of patients treated with zonisamide experienced a 75% or greater reduction, compared to approximately 12% in the placebo groups.
No differences in efficacy based on age, sex or race, as measured by a change in seizure frequency from baseline, were detected.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Administration
Inform patients that a pharmacist will provide an appropriate device and instructions for measuring the correct dose and that a household teaspoon is not an accurate measuring device. Instruct patients to shake ZONISADE well and discard any unused portion after 30 days of opening the bottle [see Dosage and Administration ( 2.2)] .
Drowsiness
ZONISADE may produce drowsiness, especially at higher doses. Patients should be advised not to drive a car or operate other complex machinery until they have gained experience on ZONISADE sufficient to determine whether it affects their performance. Because of the potential of zonisamide to cause CNS depression, as well as other cognitive and/or neuropsychiatric adverse events, ZONISADE should be used with caution if used in combination with alcohol or other CNS depressants.
Serious Skin Reactions
Patients should contact their physicians immediately if a skin rash develops [see Warnings and Precautions ( 5.2)] .
Acute Myopia and Secondary Angle Closure Glaucoma
Instruct patients to seek immediate medical attention if they experience blurred vision, visual disturbances, or periorbital pain [see Warnings and Precautions ( 5.6)] .
Kidney Stones
Patients should contact their physician immediately if they develop signs or symptoms, such as sudden back pain, abdominal pain, and/or blood in the urine, that could indicate a kidney stone. Increasing fluid intake and urine output may reduce the risk of stone formation, particularly in those with predisposing risk factors for stones [see Warnings and Precautions ( 5.15)] .
Oligohidrosis and Hyperthermia in Pediatric Patients
Patients should contact their physician immediately if a child has been taking ZONISADE and is not sweating as usual with or without a fever [see Warnings and Precautions ( 5.5)].
Serious Hematologic Events
Because zonisamide can cause hematological complications, patients should contact their physician immediately if they develop a fever, sore throat, oral ulcers, or easy bruising [see Warnings and Precautions ( 5.3)].
Suicidal Behavior and Ideation
Counsel patients and caregivers that AEDs, including ZONISADE, may increase the risk of suicidal thoughts and behavior and advise them of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers [see Warnings and Precautions ( 5.7)].
Hyperammonemia and Encephalopathy
Warn patients about the possible development of hyperammonemia with or without encephalopathy. Although hyperammonemia may be asymptomatic, clinical symptoms of hyperammonemic encephalopathy often include acute alterations in level of consciousness and/or cognitive function with lethargy and/or vomiting. Instruct patients to contact their physician if they develop unexplained lethargy, vomiting, or changes in mental status [see Warnings and Precautions ( 5.13)].
Metabolic Acidosis
Patients should contact their physician immediately if they develop fast breathing, fatigue/tiredness, loss of appetite, or irregular heartbeat or palpitations, which are possible manifestations of metabolic acidosis [see Warnings and Precautions ( 5.8)].
Pregnancy
Advise pregnant women and females of reproductive potential of the risk to a fetus. Advise pregnant women to inform their healthcare provider of a known or suspected pregnancy.
Advise women who are exposed to ZONISADE during pregnancy that there is a pregnancy registry that monitors pregnancy outcomes in women exposed to ZONISADE during pregnancy. Encourage patients to report their pregnancy to North American Antiepileptic Drug (NAAED) Pregnancy Registry at 1-888-233-2334 or http://www.aedpregnancyregistry.org/ [see Use in Specific Populations ( 8.1)].
Lactation
Advise breastfeeding women using ZONISADE to monitor infants for increased sleepiness, decreased appetite, and elevated temperature and to seek medical attention if they notice these signs [see Use in Specific Populations ( 8.2)].
Manufactured for:
Azurity Pharmaceuticals, Inc.
Wilmington, MA 01887Made in United Kingdom
Patent: https://azurity.com/patents
This product’s labeling may have been updated. For current Full Prescribing Information, please visit www.zonisade.com
PN: 65628-00630 REV#: 01 03/23
-
Medication Guide
ZONISADE ®(Zaan-i-said)(zonisamide oral suspension)
What is the most important information I should know about ZONISADE?
- ZONISADE may cause serious skin reactions that can cause death. These serious skin reactions may include a severe rash with blisters and peeling skin, especially around the mouth, nose, eyes and genitals (Stevens-Johnson syndrome). ZONISADE may also cause a rash with blisters and peeling skin over much of the body (toxic epidermal necrolysis). Call your healthcare provider right away if you develop a skin rash.
- ZONISADE can cause blood cell changes such as reduced red and white blood cell counts.Call your healthcare provider right away if you develop fever, sore throat, sores in your mouth, or easy bruising.
- ZONISADE can cause other types of allergic reactions or serious problems that may affect different parts of the body such as your liver, kidneys, heart, or blood cells.You may or may not have a rash with these types of reactions. These reactions can be very serious and can cause death. Call your healthcare provider right away if you have:
- fever
- rash
- swelling of your face
- weakness, fatigue
- severe muscle pain
- swollen lymph glands
- unusual bruising or bleeding
- yellowing of your skin or the white part of your eyes
- ZONISADE may cause decreased sweating and increased body temperature (fever).People, especially children, should be watched for signs of decreased sweating and fever, especially in hot temperatures. Some people may need to be hospitalized for this condition. If you have decreased sweating with or without a fever, call your healthcare provider right away.
-
ZONISADE may cause eye problems.Serious eye problems include:
- any sudden decrease in vision with or without eye pain and redness
- a blockage of fluid in the eye causing increased pressure in the eye (secondary angle closure glaucoma)
These eye problems can lead to permanent loss of vision if not treated.
Call your healthcare provider right away if you have any new eye symptoms, including any new problems with your vision.
- Like other antiepileptic drugs, ZONISADE may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- new or worse depression
- feeling agitated or restless
- trouble sleeping (insomnia)
- acting aggressive, being angry, or violent
- an extreme increase in activity and talking (mania)
- attempt to commit suicide
- new or worse anxiety
- panic attacks
- new or worse irritability
- acting on dangerous impulses
- other unusual changes in behavior or mood
Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
- Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop ZONISADE without first talking to a healthcare provider.
- Stopping ZONISADE suddenly can cause serious problems.
- Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus).
- ZONISADE can increase the level of acid in your blood (metabolic acidosis).If left untreated, metabolic acidosis can cause brittle or soft bones (osteoporosis, osteomalacia, osteopenia), kidney stones and can slow the rate of growth in children. Metabolic acidosis can happen with or without symptoms. Call your healthcare provider right away if you have any of these symptoms:
- fast breathing
- feel tired
- feel changes in heartbeat
- not feel hungry (loss of appetite)
- have trouble thinking clearly
Your healthcare provider should do a blood test to measure the level of acid in your blood before and during your treatment with ZONISADE.
-
ZONISADE may cause problems with thinking and alertness.ZONISADE may affect how you think and cause confusion, problems with concentration, attention, memory, or speech. ZONISADE may cause depression or psychotic symptoms (such as seeing or hearing things that are really not there), tiredness, and sleepiness.
ZONISADE can have other serious side effects. For more information ask your healthcare provider or pharmacist. Tell your healthcare provider if you have any side effect that bothers you. Be sure to read the section " What are the possible side effects of ZONISADE?”.
What is ZONISADE?
- ZONISADE is a prescription medicine that is used with other medicines to treat partial seizures in adults and children 16 years of age and older.
- It is not known if ZONISADE is safe and effective in children under 16 years of age.
Do not take ZONISADE if you:
- are allergic to sulfonamides or zonisamide.
Before taking ZONISADE, tell your healthcare provider about all your medical conditions, including if you:
- have or have had depression, mood problems or suicidal thoughts or behavior.
- have kidney problems.
- have liver problems.
- have a history of metabolic acidosis (too much acid in your blood).
- have weak, brittle bones or soft bones (osteomalacia, rickets, osteopenia, or osteoporosis).
- have a growth problem.
- are on a diet high in fat called a ketogenic diet.
- have diarrhea.
- have high blood levels of ammonia.
- are pregnant or plan to become pregnant. ZONISADE may harm your unborn baby. Women who can become pregnant should use effective birth control. Tell your healthcare provider right away if you become pregnant or think you may be pregnant while taking ZONISADE. You and your healthcare provider should decide if you should take ZONISADE while you are pregnant.
There is pregnancy registry for women who are exposed to ZONISADE during pregnancy. If you become pregnant while taking ZONISADE, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy. - are breastfeeding or plan to breastfeed. ZONISADE can pass into your breast milk. It is not known if ZONISADE in your breast milk can harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take ZONISADE. If you breastfeed while taking ZONISADE, check your baby and call your healthcare provider right away if your baby has increased sleepiness, decreased hunger, or elevated body temperature.
Tell your healthcare provider about all the medicines you take, including prescription and over-the- counter medicines, vitamins, and herbal supplements.
How should I take ZONISADE?
- Take ZONISADE exactly as your healthcare provider tells you to take it.
- ZONISADE is for oral use only.
- Your healthcare prescriber may change your dose. Do notchange your dose without talking to your healthcare provider.
- Your pharmacist will provide a measuring device and instructions for measuring the correct dose. Do not use a household teaspoon.
- Take ZONISADE 1 or 2 times each day, with or without food.
- Shake ZONISADE well each time before taking.
- If you take too much ZONISADE, call your local Poison Control Center or go to the nearest emergency room right away.
- Do notstop taking ZONISADE without talking to your healthcare provider. Stopping ZONISADE suddenly can cause serious problems. If you have epilepsy and you stop taking ZONISADE suddenly, you may have an increase in seizures, including seizures that will not stop (status epilepticus).
What should I avoid while taking ZONISADE?
- You should not drink alcohol or take other drugs that make you sleepy or dizzy while taking ZONISADE until you talk to your health care provider. ZONISADE taken with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not drive a car or operate machinery until you know how ZONISADE affects you. ZONISADE can slow your thinking and motor skills.
What are the possible side effects of ZONISADE?
ZONISADE can cause serious side effects including:See "What is the most important information I should know about ZONISADE?"
- high blood ammonia levels.High ammonia in the blood can affect your mental status, slow your alertness, make you feel tired, or cause vomiting. Call your healthcare provider right away if you develop unexplained tiredness, vomiting, slow alertness or changes in your mental status.
- kidney stones.Drink plenty of fluids while you take ZONISADE to decrease your chances of getting kidney stones. Call your healthcare provider right away if you get back pain, stomach pain, or blood in your urine.
- decrease in kidney function.ZONISADE may cause a decrease in kidney function. Your healthcare provider should do a blood test to measure your kidney function before and during treatment with ZONISADE.
The most common side effects of ZONISADE include:
- drowsiness
- dizziness
- agitation or irritability
- loss of appetite
- trouble with walking and coordination
- difficulty with memory or concentration
These are not all of the possible side effects of ZONISADE. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Azurity Pharmaceuticals, Inc. at 1-855-379-0383.
How should I store ZONISADE?
- Store ZONISADE at room temperature between 68°F to 77°F (20°C to 25°C).
- Protect ZONISADE from light.
- Throw away (discard) any unused ZONISADE 30 days after first opening the bottle.
Keep ZONISADE and all medicines out of the reach of children.
General Information about the safe and effective use of ZONISADE.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ZONISADE for a condition for which it was not prescribed. Do not give ZONISADE to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ZONISADE that is written for health professionals.
What are the ingredients in ZONISADE?
Active ingredient:zonisamideInactive ingredients:carboxymethylcellulose sodium, citric acid monohydrate, microcrystalline cellulose, purified water, sodium benzoate, strawberry flavor, sucralose, trisodium citrate dihydrate, and xanthan gum.
Manufactured for:
Azurity Pharmaceuticals, Inc.
Wilmington, MA 01887 USAThis Medication Guide has been approved by the U.S. Food and Drug Administration.
Approved: 03/2023
-
PRINCIPAL DISPLAY PANEL - Carton Label
NDC: 52652-8001-1
ZONISADE ®
(zonisamide
oral suspension)100 mg/5 mL
FOR ORAL USE ONLY
SHAKE WELL BEFORE USE150 mL Rx Only
ATTENTION PHARMACIST:
Dispense Medication Guide to each
patient. Medication Guide available at:
zonisade.com/medication-guide.pdfazurity ®
pharmaceuticals -
INGREDIENTS AND APPEARANCE
ZONISADE
zonisamide suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59368-407 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZONISAMIDE (UNII: 459384H98V) (ZONISAMIDE - UNII:459384H98V) ZONISAMIDE 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCRALOSE (UNII: 96K6UQ3ZD4) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color white (white to off-white) Score Shape Size Flavor STRAWBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59368-407-01 1 in 1 CARTON 07/15/2022 1 150 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214273 07/15/2022 Labeler - Praxis, LLC (016329513) Establishment Name Address ID/FEI Business Operations Praxis, LLC 016329513 manufacture(59368-407) , label(59368-407) , pack(59368-407)
Trademark Results [ZONISADE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZONISADE 90400843 not registered Live/Pending |
Eton Pharmaceuticals, Inc. 2020-12-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.