Sodium Sulfacetamide 9% and Sulfur 4.25% Suspension
Sodium Sulfacetamide and Sulfur by
Drug Labeling and Warnings
Sodium Sulfacetamide and Sulfur by is a Prescription medication manufactured, distributed, or labeled by Scite Pharma, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SODIUM SULFACETAMIDE AND SULFUR- sulfacetamide sodium and sulfur suspension
Scite Pharma, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Sodium Sulfacetamide 9% and Sulfur 4.25% Suspension
DESCRIPTION
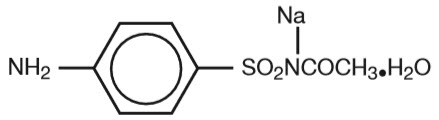
Sodium sulfacetamide is a sulfonamide with antibacterial activity while sulfur acts as a keratolytic agent. Chemically sodium sulfacetamide is N-[(4-aminophenyl) sulfonyl]-acetamide, monosodium salt, monohydrate. The structural formula is:
Each mL contains 90 mg of sodium sulfacetamide and 42.5 mg of sulfur in vehicle consisting of aloe vera gel, ammonium lauryl sulfate, butylated hydroxytoluene, cetyl alcohol, cocamidopropyl betaine, disodium EDTA, glycerin, glyceryl stearate SE, green tea extract, guar gum, methylparaben, PEG 100 stearate, propylene glycol, propylparaben, purified water, sodium thiosulfate, stearyl alcohol, and triacetin.
CLINICAL PHARMACOLOGY
Sodium sulfacetamide exerts a bacteriostatic effect against sulfonamide sensitive Gram-positive and Gram-negative microorganisms commonly isolated from secondary cutaneous pyogenic infections. It acts by restricting the synthesis of folic acid required by bacteria for growth, by its competition with para-aminobenzoic acid. There is no clinical data available on the degree and rate of systemic absorption of this product when applied to the skin or scalp. However, significant absorption of sodium sulfacetamide through the skin has been reported.
The following in vitro data is available but the clinical significance is unknown. Organisms that show susceptibility to sodium sulfacetamide are: Streptococci, Staphylococci, E. coli, Klebsiella pneumoniae, Pseudomonas pyocyanea, Salmonella species, Proteus vulgaris, Nocardia and Actinomyces.
The exact mode of action of sulfur in the treatment of acne is unknown, but it has been reported that it inhibits the growth of Propionibacterium acnes and the formation of free fatty acids.
INDICATIONS
Sodium Sulfacetamide 9% and Sulfur 4.25% Suspension is indicated in the topical control of acne vulgaris, acne rosacea and seborrheic dermatitis.
CONTRAINDICATIONS
Sodium Sulfacetamide 9% and Sulfur 4.25% Suspension is contraindicated for use by patients having known hypersensitivity to sulfonamides, sulfur or any other component of this preparation. Clenia Plus is not to be used by patients with kidney disease.
WARNINGS
Although rare, sensitivity to sodium sulfacetamide may occur. Therefore, caution and careful supervision should be observed when prescribing this drug for patients who may be prone to hypersensitivity to topical sulfonamides. Systemic toxic reactions such as agranulocytosis, acute hemolytic anemia, purpura hemorrhagica, drug fever, jaundice and contact dermatitis indicate hypersensitivity to sulfonamides. Particular caution should be employed if areas of denuded or abraded skin are involved.
Sulfonamides are known to cause Stevens-Johnson syndrome in hypersensitive individuals. Stevens-Johnson syndrome also has been reported following the use of sodium sulfacetamide topically. Cases of drug-induced systemic lupus erythematosus from topical sulfacetamide also have been reported. In one of these cases, there was a fatal outcome.
FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.Avoid contact with eyes, lips and mucous membranes.
KEEP OUT OF REACH OF CHILDREN.Keep container tightly closed.
PRECAUTIONS
General
Nonsusceptible organisms, including fungi, may proliferate with the use of this preparation. If irritation develops, use of the product should be discontinued and appropriate therapy instituted. Patients should be carefully observed for possible local irritation or sensitization during long-term therapy. Systemic absorption of topical sulfonamides is greater following application to large, infected, abraded, denuded or severely burned areas. Under these circumstances, any of the adverse effects produced by the systemic administration of these agents could potentially occur, and appropriate observations and laboratory determinations should be performed.
The object of this therapy is to achieve desquamation without irritation, but sodium sulfacetamide and sulfur can cause reddening and scaling of the epidermis. These side effects are not unusual in the treatment of acne vulgaris, but patients should be cautioned about the possibility.
Information for patients
Avoid contact with eyes, lips and mucous membranes. Patients should discontinue the use of this product if the condition becomes worse or if a rash develops in the area being treated or elsewhere. The use of this product should also be discontinued promptly and the physician notified if any arthritis, fever or sores in the mouth develop.
Carcinogenesis, Mutagenesis and Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential. Studies on reproduction and fertility also have not been performed. Chromosomal nondisjunction has been reported in the yeast, Saccharomyces cerevisiae, following application of sodium sulfacetamide. The significance of this finding to the topical use of sodium sulfacetamide in the human is unknown.
PREGNANCY
Category C
Animal reproduction studies have not been conducted with Sodium Sulfacetamide 9% and Sulfur 4.25% Suspension. It is not known whether Sodium Sulfacetamide 9% and Sulfur 4.25% Suspension can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Clenia Plus should be given to a pregnant woman only if clearly needed or when potential benefits outweigh potential hazards to the fetus.
NURSING MOTHERS
It is not known whether this drug is excreted in the human milk. However, small amounts of orally administered sulfonamides have been reported to be eliminated in human milk. In view of this and because many drugs are excreted in human milk, caution should be exercised when Sodium Sulfacetamide 9% and Sulfur 4.25% Suspension is administered to a nursing woman.
ADVERSE REACTIONS
Reports of irritation and hypersensitivity to sodium sulfacetamide are uncommon. The following adverse reactions, reported after administration of sterile ophthalmic sodium sulfacetamide, are noteworthy: instances of Stevens-Johnson syndrome and instances of local hypersensitivity which progressed to a syndrome resembling systemic lupus erythematosus; in one case a fatal outcome was reported (see WARNINGS).
Call your doctor for medical advice about side effects. To report SUSPECTED ADVERSE REACTIONSor obtain product information, contact Scite Pharma, LLC at 1-866-633-9033 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
The oral LD 50of sulfacetamide in mice is 16.5 g/kg. In the event of overdosage, emergency treatment should be started immediately.
Manifestations
Overdosage may cause nausea and vomiting. Large oral overdosage may cause hematuria, crystalluria and renal shutdown due to the precipitation of sulfa crystals in the renal tubules and the urinary tract. For treatment, contact your local Poison Control Center (1-800-222-1222), or your doctor.
DOSAGE AND ADMINISTRATION
SHAKE WELL before use.Cleanse affected areas. Apply Sodium Sulfacetamide 9% and Sulfur 4.25% Suspension once or twice daily to affected areas, or as directed by your physician. Wet skin and liberally apply to areas to be cleansed. Massage gently into skin for 10-20 seconds, working into a full lather, rinse thoroughly and pat dry. If skin dryness occurs, it may be controlled by rinsing off Sodium Sulfacetamide 9% and Sulfur 4.25% Suspension sooner or using less often.
HOW SUPPLIED
Sodium Sulfacetamide 9% and Sulfur 4.25% Suspension is available in 8 fl oz (237 mL) bottles, NDC: 79043-980-08.
Manufactured for:
Scite Pharma, LLC
Canton, MS 39046
Manufactured in U.S.A
Rev. 12/2021
All prescriptions using this product shall be made subject to state and federal statutes as applicable. NOTE: this is not an Orange Book product and has not been subjected to FDA therapeutic equivalency or other equivalency testing.
PRINCIPAL DISPLAY PANEL - 237 mL Bottle Label
NDC: 79043-980-08
SODIUM
SULFACETAMIDE 9%
AND SULFUR 4.25%
SUSPENSION
Topical Suspension in a vehicle
containing Green Tea and Aloe
FOR EXTERNAL USE ONLY
NOT FOR OPHTHALMIC USE
SHAKE WELL Rx Only
scite
PHARMA
Net Wt. (8 oz) 237 mL

| SODIUM SULFACETAMIDE AND SULFUR
sulfacetamide sodium and sulfur suspension |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Scite Pharma, LLC (117555106) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.