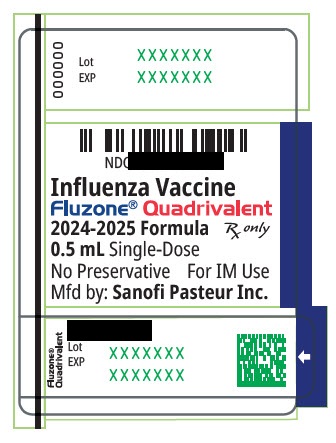
These highlights do not include all the information needed to use Fluzone ®Quadrivalent safely and effectively. See full prescribing information for Fluzone Quadrivalent. Fluzone Quadrivalent (Influenza Vaccine) Injectable Suspension, for Intramuscular Use 2024-2025 Formula Initial U.S. Approval: 2013
Fluzone Quadrivalent Northern Hemisphere by
Drug Labeling and Warnings
Fluzone Quadrivalent Northern Hemisphere by is a Other medication manufactured, distributed, or labeled by Bamboo US BidCo LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FLUZONE QUADRIVALENT NORTHERN HEMISPHERE- influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/california/122/2022 san-022 (h3n2) antigen (formaldehyde inactivated), influenza b virus b/phuket/3073/2013 antigen (formaldehyde inactivated), and influenza b virus b/michigan/01/2021 antigen (formaldehyde inactivated) injection, suspension
Bamboo US BidCo LLC
----------
These highlights do not include all the information needed to use Fluzone
®Quadrivalent safely and effectively. See full prescribing information for Fluzone Quadrivalent.
Fluzone Quadrivalent (Influenza Vaccine)
Injectable Suspension, for Intramuscular Use
2024-2025 Formula
Initial U.S. Approval: 2013
| FLUZONE QUADRIVALENT NORTHERN HEMISPHERE
influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/california/122/2022 san-022 (h3n2) antigen (formaldehyde inactivated), influenza b virus b/phuket/3073/2013 antigen (formaldehyde inactivated), and influenza b virus b/michigan/01/2021 antigen (formaldehyde inactivated) injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bamboo US BidCo LLC (119087615) |
Revised: 6/2025
Document Id: 37f16746-cfaa-daa0-e063-6294a90a8062
Set id: 37f1703f-dec0-a588-e063-6294a90afb89
Version: 1
Effective Time: 20250619
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.