Fluzone (Influenza Vaccine) injectable suspension, for intramuscular use 2025 Formula Initial U.S. Approval: 1980 These highlights do not include all the information needed to use Fluzone ®Southern Hemisphere safely and effectively. See full prescribing information for Fluzone Southern Hemisphere.
FLUZONE TRIVALENT SOUTHERN HEMISPHERE by
Drug Labeling and Warnings
FLUZONE TRIVALENT SOUTHERN HEMISPHERE by is a Other medication manufactured, distributed, or labeled by Bamboo US BidCo LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
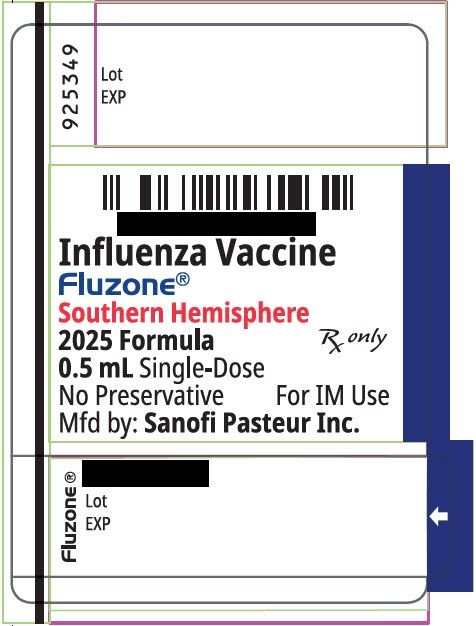
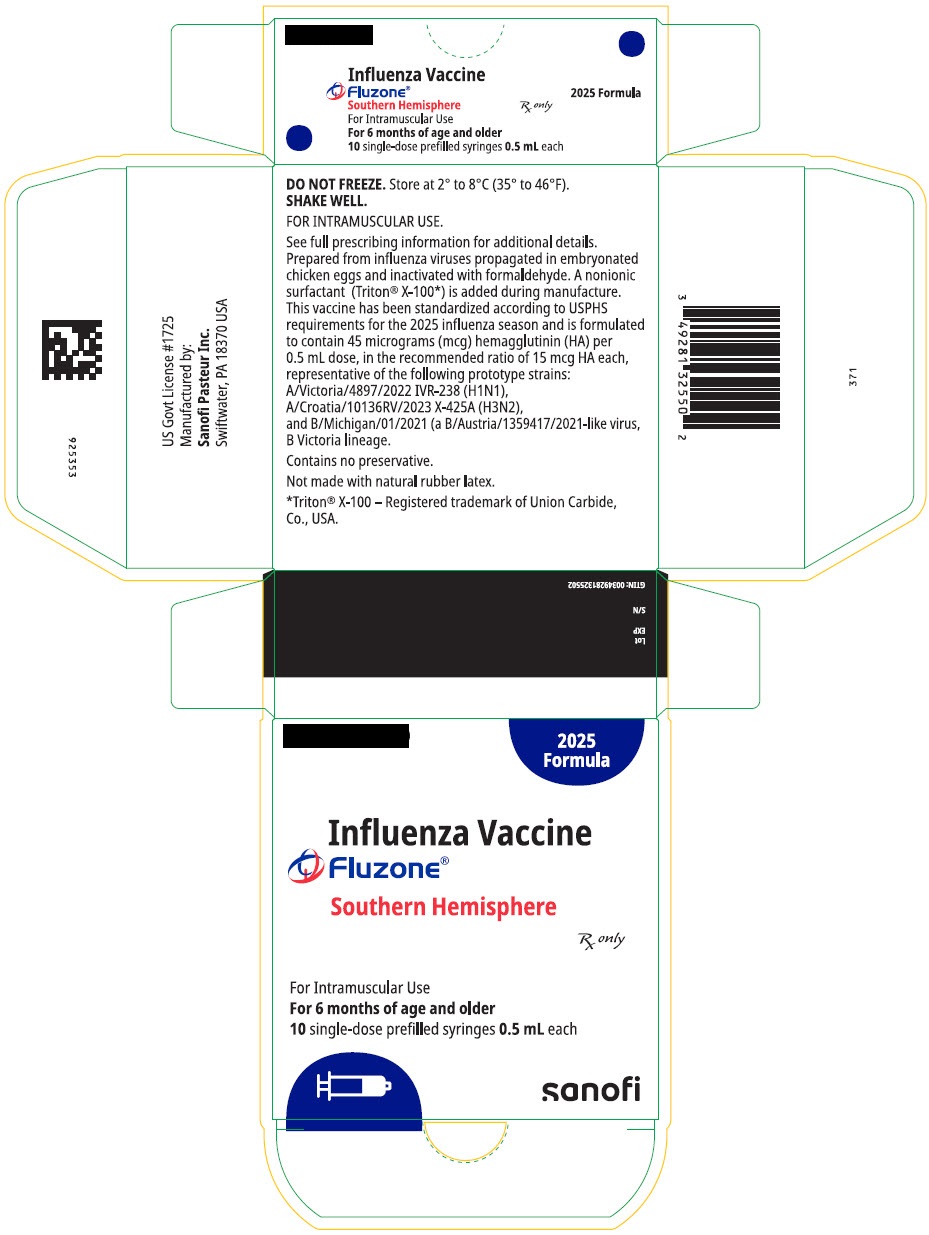
FLUZONE TRIVALENT SOUTHERN HEMISPHERE- influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/croatia/10136rv/2023 x-425a (h3n2) antigen (formaldehyde inactivated), and influenza b virus b/michigan/01/2021 antigen (formaldehyde inactivated) injection, suspension
Bamboo US BidCo LLC
----------
Fluzone (Influenza Vaccine) injectable suspension, for intramuscular use 2025 Formula
Initial U.S. Approval: 1980
These highlights do not include all the information needed to use Fluzone
®Southern Hemisphere safely and effectively. See full prescribing information for Fluzone Southern Hemisphere.
| FLUZONE TRIVALENT SOUTHERN HEMISPHERE
influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/croatia/10136rv/2023 x-425a (h3n2) antigen (formaldehyde inactivated), and influenza b virus b/michigan/01/2021 antigen (formaldehyde inactivated) injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bamboo US BidCo LLC (119087615) |
Revised: 6/2025
Document Id: 37f1a8d4-30e8-faa5-e063-6294a90a6650
Set id: 37f1a8cb-1d95-0ed1-e063-6294a90a631d
Version: 1
Effective Time: 20250619
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.