ANASED- xylazine injection, solution
Xylazine by
Drug Labeling and Warnings
Xylazine by is a Animal medication manufactured, distributed, or labeled by Cronus Pharma LLC, Cronus Pharma Specialities India Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION:
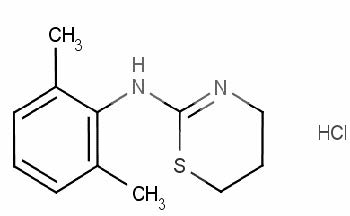
Xylazine is an alpha2-adrenoreceptor agonist with sedative properties. The chemical name for Xylazine is 2-(2,6-dimethylphenylamino)-4H-5,6-dihydro-1,3-thiazine. The formula for the hydrochloride salt is C12H16N2S.HCl. Anased® Equine Injection (xylazine injection) is a clear, colorless solution. Each mL of sterile Anased® Equine Injection contains xylazine HCl equivalent to 100 mg xylazine base; 0.9 mg methylparaben and 0.1 mg propylparaben as preservatives; 6.12 mg sodium citrate dihydrate; water for injection, q.s. hydrochloric acid and/or sodium citrate dihydrate may also be used as necessary to adjust the pH.
-
INDICATIONS & USAGE
INDICATIONS: Anased® Equine Injection should be used in horses when it is desirable to produce a state of sedation. It has been successfully used when conducting various diagnostic, orthopedic and dental procedures of short duration. It may also be used as a preanesthetic to local or general anesthesia.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION: For intravenous or intramuscular administration in horses. The recommended dosage for intravenous (IV) administration is 0.5 mL/100 lbs body weight (0.5 mg/lb). The recommended dosage for intramuscular (IM) administration is 1 mL/100lbs body weight (1 mg/lb).
Following the administration of Anased® Equine Injection the horse should be allowed to rest quietly until the full effect has been reached. These dosages produce a state of sedation which is usually maintained for 1 to 2 hours (See Clinical Pharmacology).
Preanesthetic to local anesthesia: At the recommended dosages Anased® Equine Injection may be used in conjunction with local anesthetics, such as procaine and lidocaine.
Preanesthetic to general anesthesia: At the recommended dosages Anased® Equine Injection produces an additive effect to central nervous system depressants, such as sodium pentobarbital, sodium thiopental and sodium thiamylal. Accordingly, the dosage of such products should be reduced and administered to the desired effect. Generally, 1/3 to 1/2 of the calculated dosage of the barbiturates will be needed to produce a surgical plane of anesthesia. Postanesthetic or emergence excitement has not been observed in horses preanesthetized with Anased® Equine Injection.
Anased® Equine Injection has been successfully used as a preanesthetic agent for sodium pentobarbital, sodium thiopental, sodium thiamylal, nitrous oxide, ether, halothane, guaifenesin and methoxyflurane anesthesia.
- CONTRAINDICATIONS
-
WARNINGS:User Safety Warnings
Not for use in humans. Keep out of reach of children.
Avoid skin, eye or mucosal contact. Use caution while handling and using filled syringes.
Absorption of the active ingredients is possible following exposure via the skin, eye or mucosa. In case of accidental eye exposure, flush eyes with water for 15 minutes, remove contact lenses then continue to flush. In case of accidental skin exposure, wash with soap and water and remove contaminated clothing. If symptoms occur, seek the advice of a physician.
In case of accidental oral intake or self-injection, seek medical advice immediately and show the package insert to the physician. DO NOT DRIVE as sedation, loss of consciousness, and changes in blood pressure may occur.
Persons with cardiovascular disease (for example, hypertension or ischemic heart disease) should take special precautions to avoid any exposure to this product.
Pregnant women should exercise special caution to avoid exposure. Uterine contractions and decreased fetal blood pressure may occur after accidental systemic exposure.
Persons with known hypersensitivity to any of the ingredients should avoid contact with Anased® Equine Injection.
Caution should be exercised when handling sedated animals. Handling or any other sudden stimuli, including noise, may cause a defense reaction in an animal that appears to be heavily sedated.
- SPL UNCLASSIFIED SECTION
-
Animal Safety Warnings
Intracarotid arterial injection should be avoided. As with many drugs, including tranquilizers, immediate and violent seizures followed by collapse may result from inadvertent administration into the carotid artery. Although the reaction with Anased® Equine Injection is usually transient and the recovery rapid and complete, special care should be taken to assure that the needle is in the jugular vein rather than the carotid artery.
- Other Warnings
-
PRECAUTIONS:
Debilitated horses with depressed respiration, cardiac disease, renal or liver impairment, shock or any other stress conditions should be carefully monitored whenever Anased® Equine Injection is administered.
Anased® Equine Injection produces an additive effect to central nervous system depressants and caution should be taken when administering barbiturate compounds in conjunction with Anased® Equine Injection. Barbiturates should be administered at a reduced dosage and to the desired effect, and when injected intravenously should be given slowly.
Arrhythmias resulting in partial atrioventricular (AV) blocks and bradycardia are transient changes which may occur, but that can be counteracted to a large degree by the administration of atropine prior to or following Anased® Equine Injection (xylazine injection).
Analgesic effect is variable and depth should be carefully determined prior to surgical or clinical procedures. Variability of analgesia occurs most frequently at the distal extremities of horses. In spite of sedation, the practitioner should proceed with caution since defense reactions may not be diminished.
Horses under the influence of Anased® Equine Injection are particularly sensitive to noise and care should be taken accordingly to avoid risk of injury. Sedation for transport is most successful if actual transport is initiated after full effect of the drug has been obtained and the horse’s stability maintained in the standing position.
-
ADVERSE REACTIONS
ADVERSE REACTIONS: The deep sedation produced by Anased® Equine Injection is characterized by lowering of the head, drooping of the eyelids and lower lip and a marked reluctance to move. Most horses given Anased® Equine Injection sweat around the ears and poll region and may seem particularly sensitive to sharp auditory stimuli during the recovery period. The respiratory and pulse rate are reduced as in natural sleep. Transient changes in the conductivity of the cardiac muscle (resulting in partial AV blocks), bradycardia, decreased cardiac output and a rise in arterial blood pressure may occur following intravenous administration.
-
SPL UNCLASSIFIED SECTION
CONTACT INFORMATION: To report suspected adverse reactions, for technical assistance, or to obtain a copy of the Safety Data Sheet (SDS), call Cronus Pharma at (844) 227-6687. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY: Xylazine Injection is pharmacologically classified as a non-narcotic sedative. The drug causes sedation by acting upon alpha-adrenergic receptors in the brain to prevent the release of norepinephrine. Xylazine Injection also produces muscle relaxation by inhibiting the intraneural transmission of impulses in the central nervous system.
Deep sedation develops in the horse within 10 to 15 minutes after intramuscular injection, and within 3 to 5 minutes following intravenous administration.
Deep sedation lasts 15 to 20 minutes, while a sleep-like state, the depth of which is dose-dependent, is usually maintained for 1 to 2 hours following intramuscular administration of the drug at the recommended dosage. Recovery is complete with 30-40 minutes following intravenous injection.
In animals under the influence of Xylazine Injection, the respiratory and pulse rates are reduced as in a natural sleep. The intramuscular injection of Xylazine produces only negligible effects on the cardiovascular and respiratory systems. However, intravenous administration in the horse may cause transient changes in the conductivity of the cardiac muscle, evidenced by partial AV blocks, bradycardia, decreased cardiac output and a rise in arterial blood pressure. These actions are transient and can be counteracted to a large degree by the administration of atropine prior to or following Xylazine Injection. Xylazine Injection has no effect on blood clotting time or other hematologic parameters.
- SPL UNCLASSIFIED SECTION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANASED
xylazine injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 69043-043 Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength XYLAZINE HYDROCHLORIDE (UNII: NGC3S0882S) (XYLAZINE - UNII:2KFG9TP5V8) XYLAZINE 100 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69043-043-05 1 in 1 CARTON 1 50 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA140442 02/12/2024 Labeler - Cronus Pharma LLC (079421067) Registrant - Cronus Pharma Specialities India Private Limited (876818318) Establishment Name Address ID/FEI Business Operations Cronus Pharma Specialities India Private Limited 876818318 analysis, manufacture, label, pack
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
