Micuraderm by Aletris Pharmaceuticals, LLC MICURADERM-
Micuraderm by
Drug Labeling and Warnings
Micuraderm by is a Other medication manufactured, distributed, or labeled by Aletris Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Product Description:
-
Indications for Use:
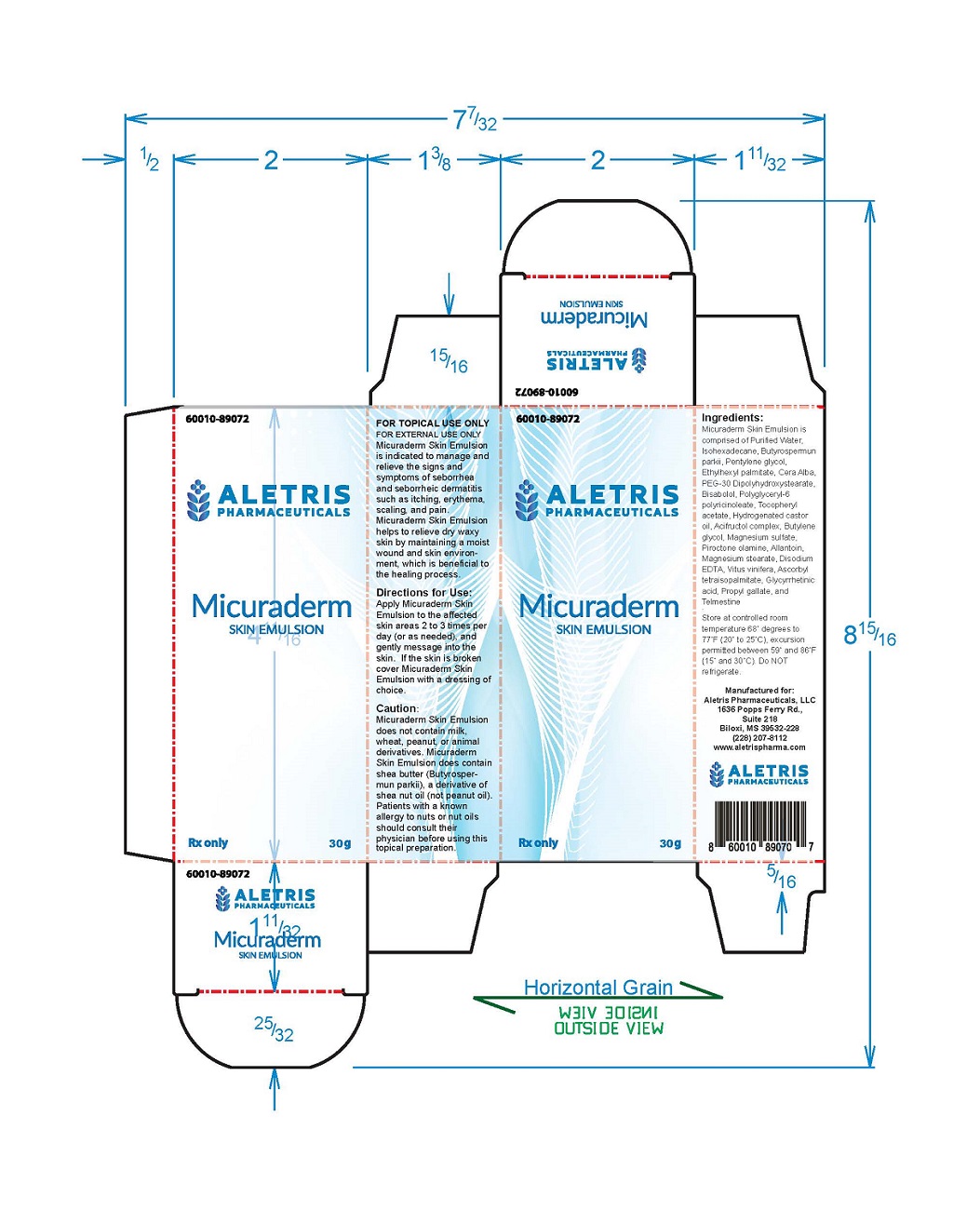
Under the supervision of a healthcare professional, Micuraderm Skin Emulsion is indicated to manage and relieve the signs and symptoms of seborrhea and seborrheic dermatitis such as itching, erythema, scaling, and pain. Micuraderm Skin Emulsion helps to relieve dry waxy skin by maintaining a moist wound and skin environment, which is beneficial to the healing process.
- Directions for Use:
-
Ingredients:
Micuraderm Skin Emulsion is comprised of Purified Water, lsohexadecane, Butyrospermun parkii, Pentylene glycol, Ethylhexyl palmitate, Cera Alba, PEG-30 Dipolyhydroxystearate, Bisabolol, Polyglyceryl-6 polyricinoleate, Tocopheryl acetate, Hydrogenated castor oil, Acifructol complex, Butylene glycol, Magnesium sulfate, Piroctone olamine, Allantoin, Magnesium stearate, Disodium EDTA, Vitus vinifera, Ascorbyl tetraisopalmitate, Glycyrrhetinic acid, Propyl gallate, and Telmestine.
-
Caution:
The use of Micuraderm Skin Emulsion is contraindicated in any patient with a known history of hypersensitivity to any of the ingredients. Micuraderm Skin Emulsion does not contain milk, wheat, peanut, or animal derivatives. Micuraderm Skin Emulsion does contain shea butter (Butyrospermun parkii), a derivative of shea nut oil (not peanut oil). Patients with a known allergy to nuts or nut oils should consult their physician before using this topical preparation.
-
How Supplied:
30g Tube
To Open: Puncture seal with pointed end of cap.
Important: The opening of this product is covered with a metal seal. Do no use If seal has been punctured or is not visible.
Store at controlled room temperature 68°degrees to 77°F (20° to 25°C), excursion permitted between 59° and 86°F (15° and 30°C). Do NOT refrigerate.
Manufactured for:
Aletris Pharmaceuticals, LLC
1636n Papps Ferry Rd., Suite 218
Biloxi, MS 39532-228
(228) 207-8112
www.aletrispharma.com
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MICURADERM
dressing, wound, drugProduct Information Product Type PRESCRIPTION MEDICAL DEVICE Item Code (Source) GS1:86001089072 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 GS1:86001089072 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date premarket notification K111168 10/28/2023 Labeler - Aletris Pharmaceuticals, LLC (118956541)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.