RYALTRIS- olopatadine hydrochloride and mometasone furoate spray, metered
Ryaltris by
Drug Labeling and Warnings
Ryaltris by is a Prescription medication manufactured, distributed, or labeled by HIKMA SPECIALTY USA INC., Glenmark Specialty SA, Renaissance Lakewood LLC, Glenmark Pharmaceuticals Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use RYALTRIS® safely and effectively. See full prescribing information for RYALTRIS.
RYALTRIS (olopatadine hydrochloride and mometasone furoate monohydrate nasal spray)
Initial U.S. Approval: 2022INDICATIONS AND USAGE
RYALTRIS is a combination of olopatadine, a histamine-1 (H1)-receptor inhibitor, and mometasone furoate, a corticosteroid, indicated for the treatment of symptoms of seasonal allergic rhinitis in adult and pediatric patients 12 years of age and older. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Nasal spray: 665 mcg of olopatadine hydrochloride and 25 mcg of mometasone furoate in each spray. (3)
CONTRAINDICATIONS
Patients with known hypersensitivity to any ingredients of RYALTRIS, including mometasone furoate. (4)
WARNINGS AND PRECAUTIONS
- Epistaxis, nasal ulcerations, nasal septal perforations, impaired wound healing, and Candida albicans infection: Monitor patients periodically for signs of adverse reactions on the nasal mucosa. (5.1)
- Somnolence: Avoid engaging in hazardous occupations requiring complete mental alertness and motor coordination such as driving or operating machinery when taking RYALTRIS. (5.2)
- Avoid concurrent use of alcohol or other central nervous system (CNS) depressants with RYALTRIS because additional reductions in alertness and additional impairment of CNS performance may occur. (5.2)
- Glaucoma and cataracts: Monitor patients with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts. (5.3)
- Hypersensitivity Reactions: Hypersensitivity reactions can occur with RYALTRIS. Hypersensitivity reactions including wheezing, have occurred after the nasal administration of mometasone furoate. Discontinue RYALTRIS if such reactions occur. (5.4)
- Immunosuppression and Risk of Infections: Potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex. More serious or even fatal course of chickenpox or measles in susceptible patients: Use caution in patients with the above because of the potential for worsening of these infections. (5.5)
- Hypercorticism and adrenal suppression with misuse or use of higher-than-recommended dosages or at the regular dosage in susceptible patients at risk for such effects (5.6)
- Potential reduction in growth velocity in children: Routinely monitor the growth in pediatric patients receiving RYALTRIS. (5.7, 8.4)
ADVERSE REACTIONS
The most common adverse reactions (≥1% incidence) are dysgeusia, epistaxis, and nasal discomfort. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Hikma Specialty USA Inc. at 1-800-962-8364 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Local Nasal Adverse Reactions
5.2 Somnolence and Impaired Mental Alertness
5.3 Glaucoma and Cataracts
5.4 Hypersensitivity Reactions
5.5 Immunosuppression and Risk of Infections
5.6 Hypercorticism and Adrenal Suppression
5.7 Effect on Growth
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Central Nervous System Depressants
7.2 Inhibitors of Cytochrome P450 3A4
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For nasal use only.
The recommended dosage of RYALTRIS is 2 sprays (2 sprays deliver a total of 1,330 mcg of olopatadine hydrochloride and 50 mcg of mometasone furoate) in each nostril twice daily.
- Shake the bottle well before each use.
- Prime RYALTRIS before initial use by releasing 6 sprays or until a fine mist appears. When RYALTRIS has not been used for 14 or more days, re-prime by releasing 2 sprays or until a fine mist appears.
- Avoid spraying RYALTRIS into the eyes or mouth.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
RYALTRIS is contraindicated in patients with known hypersensitivity to any ingredients of RYALTRIS. Hypersensitivity reactions, including wheezing, has occurred after nasal administration of mometasone furoate [see Warnings and Precautions (5.4)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Local Nasal Adverse Reactions
Epistaxis
Epistaxis was observed in 1% of patients treated with RYALTRIS and 0.6% of patients who received placebo in 2-week studies in patients with seasonal allergic rhinitis [see Adverse Reactions (6.1)].
Nasal Ulceration and Nasal Septal Perforation
Instances of nasal ulceration and nasal septal perforation have occurred in patients following the nasal application of antihistamines such as RYALTRIS.
Monitor patients periodically for signs of adverse effects on the nasal mucosa.
Impaired Nasal Wound Healing
Because of the inhibitory effect of corticosteroids on wound healing, patients who have experienced recent nasal septal ulcers, nasal surgery, or nasal trauma should avoid use of RYALTRIS until healing has occurred.
Local Candida Infection
Localized infections of the nose and pharynx with Candida albicans have occurred from nasal administration of mometasone furoate.
When such an infection occurs, discontinue RYALTRIS and institute appropriate local or systemic therapy. Patients using RYALTRIS over several months or longer should be examined periodically for evidence of Candida infection.
5.2 Somnolence and Impaired Mental Alertness
Patients should be cautioned against engaging in hazardous occupations requiring complete mental alertness and motor coordination, such as operating machinery or driving a motor vehicle, after administration of RYALTRIS. Concurrent use of RYALTRIS with alcohol or other central nervous system (CNS) depressants should be avoided because additional reductions in alertness and additional impairment of CNS performance may occur.
Somnolence was reported in 0.3% of patients treated with RYALTRIS and none of the patients who received placebo in 2-week studies in patients with seasonal allergic rhinitis [see Adverse Reactions (6.1)].
5.3 Glaucoma and Cataracts
Nasal and inhaled corticosteroids including RYALTRIS can result in the development of glaucoma and/or cataracts. Therefore, close monitoring is warranted in patients with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts.
5.4 Hypersensitivity Reactions
Hypersensitivity reactions can occur with RYALTRIS. Hypersensitivity reactions including wheezing, have occurred after the nasal administration of mometasone furoate. Discontinue RYALTRIS if such reactions occur [see Contraindications (4)].
5.5 Immunosuppression and Risk of Infections
Persons who are using drugs that suppress the immune system, such as corticosteroids, including RYALTRIS, are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The safety and effectiveness of RYALTRIS have not been established in pediatric patients less than 12 years of age and RYALTRIS is not indicated for use in this population. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated (see the respective Prescribing Information for VZIG and IG). If chickenpox develops, treatment with antiviral agents may be considered.
Corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculous infections of the respiratory tract, untreated local or systemic fungal or bacterial infections, systemic viral or parasitic infections, or ocular herpes simplex because of the potential for worsening of these infections.
5.6 Hypercorticism and Adrenal Suppression
Hypercorticism and adrenal suppression may occur when nasal corticosteroids, including RYALTRIS, are misused by taking higher-than-recommended dosages [see Dosage and Administration (2)] or in patients at risk for such effects.
5.7 Effect on Growth
Nasal corticosteroids, including RYALTRIS, may cause a reduction in growth velocity when administered to pediatric patients. The safety and effectiveness of RYALTRIS have not been established in pediatric patients less than 12 years of age and RYALTRIS is not indicated for use in this population. Routinely monitor the growth of pediatric patients receiving RYALTRIS [see Use in Specific Populations (8.4)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
- Local Nasal Adverse Reactions [see Warnings and Precautions (5.1)]
- Somnolence and Impaired Mental Alertness [see Warnings and Precautions (5.2)]
- Glaucoma and Cataracts [see Warnings and Precautions (5.3)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.4)]
- Immunosuppression and Risk of Infections [see Warnings and Precautions (5.5)]
- Hypercorticism and Adrenal Suppression [see Warnings and Precautions (5.6), Use in Specific Populations (8.4)]
- Effect on Growth [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared with rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Adults and Pediatric Patients 12 Years of Age and Older
The pooled RYALTRIS safety population reflects exposure to RYALTRIS at 2 sprays (2 sprays deliver a total of 1,330 mcg of olopatadine hydrochloride and 50 mcg of mometasone furoate) in each nostril twice daily in a total of 1189 patients from Studies 1 and 2 [see Clinical Studies (14)] and from three additional placebo and/or active-controlled studies in patients with allergic rhinitis. One placebo controlled study was a 52-week safety study. In this study, 393 patients were exposed to RYALTRIS for one year, and no new safety signals were observed.
The RYALTRIS safety population described below reflects exposure to RYALTRIS at 2 sprays (2 sprays deliver a total of 1,330 mcg of olopatadine hydrochloride and 50 mcg of mometasone furoate) in each nostril twice daily for two weeks duration in a total of 789 patients, including 596 patients from Studies 1 and 2 [see Clinical Studies (14)], and 36 and 157 from two additional placebo and active-controlled studies in patients with seasonal allergic rhinitis. The demographics of the RYALTRIS-treated patients were 12 to 81 years of age (mean age of 40 years; 67% female; 81% White, 15% Black/African American and 3% Other).
Table 1 lists adverse reactions from the safety population reported with frequencies ≥1% and more frequently than placebo in patients treated with RYALTRIS. Somnolence was reported in <1% (2 of 789) of patients treated with RYALTRIS and no patients treated with placebo.
Table 1: Adverse Reactions with ≥1% Incidence that Were Reported More Frequently with RYALTRIS than Placebo in the Safety Population in Adult and Pediatric Patients 12 Years of Age and Older with Seasonal Allergic Rhinitis RYALTRIS
N=789
n (%)Olopatadine HCl Nasal Spray*
N=751
n (%)Mometasone Furoate Nasal Spray*
N=746
n (%)Placebo
N=776
n (%)Dysgeusia
24 (3.0)
16 (2.1)
0 (0)
2 (0.3)
Epistaxis
8 (1.0)
11 (1.5)
6 (0.8)
5 (0.6)
Nasal discomfort
8 (1.0)
4 (0.5)
4 (0.5)
6 (0.8)
* Non-US approved drugs
-
7 DRUG INTERACTIONS
No formal drug-drug interaction studies have been performed with RYALTRIS. The drug interactions of the combination are expected to reflect those of the individual components [see Clinical Pharmacology (12.3)].
7.1 Central Nervous System Depressants
Concurrent use of RYALTRIS with alcohol or other central nervous system depressants should be avoided because somnolence and impairment of central nervous system performance may occur [see Warnings and Precautions (5.2)].
7.2 Inhibitors of Cytochrome P450 3A4
Studies have shown that mometasone furoate, a component of RYALTRIS, is primarily and extensively metabolized to multiple metabolites. In vitro studies have confirmed the primary role of cytochrome P450 (CYP) 3A4 in the metabolism of this compound.
Concomitant administration of CYP3A4 inhibitors may inhibit the metabolism of, and increase the mometasone furoate plasma concentration and potentially increase the risk for adverse reactions. Caution should be exercised when considering the coadministration of RYALTRIS with strong CYP3A4 inhibitors [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on RYALTRIS or mometasone furoate use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes.
Postmarketing experience with antihistamines, with similar mechanism of action to olopatadine, have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. However, there are no published human data specific to olopatadine.
Animal reproduction studies have not been conducted with RYALTRIS. However, animal reproduction studies are available for olopatadine hydrochloride and mometasone furoate. Oral administration of olopatadine hydrochloride to pregnant rats and rabbits caused a decrease in the number of live fetuses at maternal doses approximately 120 and 1600 times the maximum recommended human daily intranasal dose (MRHDID) on a mg/m2 basis, respectively (see Data). In animal reproduction studies with pregnant mice, rats, or rabbits, mometasone furoate caused increased fetal malformations and decreased fetal survival and growth following administration of doses that produced exposures approximately 1 to 16 times the MRHDID on a mcg/m2 or AUC basis (see Data). However, experience with oral corticosteroids suggests that rodents are more prone to teratogenic effects from corticosteroid exposure than humans.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
No reproductive toxicology studies were conducted with RYALTRIS; however, studies are available for olopatadine hydrochloride and mometasone furoate, as described below.
Olopatadine hydrochloride
In an oral embryo-fetal development study, pregnant rats were dosed throughout the period of organogenesis at doses up to 600 mg/kg/day. Maternal toxicity, producing death and reduced maternal body weight gain was observed at 600 mg/kg/day (approximately 1200 times the MRHDID on a mg/m2 basis). Olopatadine produced cleft palate at 60 mg/kg/day (approximately 120 times the MRHDID on a mg/m2 basis) and decreased embryo-fetal viability and reduced fetal weight in rats at 600 mg/kg/day (approximately 1200 times the MRHDID on a mg/m2 basis).
In an oral embryo-fetal development study, pregnant rabbits were dosed throughout the period of organogenesis at doses up to 400 mg/kg/day. A decrease in the number of live fetuses was observed at 400 mg/kg/day (approximately 1600 times the MRHDID on a mg/m2 basis).
In peri-/post-natal toxicity studies, pregnant rats received oral doses of olopatadine up to 600 mg/kg/day during late gestation and throughout the lactation period. Olopatadine produced decreased neonatal survival at 60 mg/kg/day (approximately 120 times the MRHDID on a mg/m2 basis) and reduced body weight gain in pups at 4 mg/kg/day (approximately 7 times the MRHDID on a mg/m2 basis). These effects appeared attributable to exposure of pups via the milk as demonstrated in a cross-fostered study in which pups of untreated dams cross-fostered to dams treated with 60 mg/kg/day olopatadine orally during the lactation period exhibited decreased body weight gain.
Mometasone furoate
In an embryo-fetal development study with pregnant mice dosed throughout the period of organogenesis, mometasone furoate produced cleft palate at a dose approximately equivalent to the MRHDID (on a mcg/m2 basis with maternal subcutaneous doses of 60 mcg/kg and above) and decreased fetal survival at approximately 4 times the MRHDID (on a mcg/m2 basis with a maternal subcutaneous dose of 180 mcg/kg). No toxicity was observed with a dose that produced an exposure approximately one-half of the MRHDID (on a mcg/m2 basis with maternal topical dermal doses of 20 mcg/kg and above).
In an embryo-fetal development study with pregnant rats dosed throughout the period of organogenesis, mometasone furoate produced fetal umbilical hernia at exposures approximately 20 times the MRHDID (on a mcg/m2 basis with maternal topical dermal doses of 600 mcg/kg and above) and delays in fetal ossification at a dose approximately 12 times the MRHDID (on a mcg/m2 basis with maternal topical dermal doses of 300 mcg/kg and above).
In another reproductive toxicity study, pregnant rats were dosed with mometasone furoate throughout pregnancy or late in gestation. Treated animals had prolonged and difficult labor, fewer live births, lower birth weight, and reduced early pup survival at a dose that was approximately equivalent to the MRHDID (on a mcg/m2 basis with a maternal subcutaneous dose of 15 mcg/kg). There were no findings at a dose approximately equivalent to or less than the MRHDID (on a mcg/m2 basis with a maternal subcutaneous dose of 7.5 mcg/kg).
Embryo-fetal development studies were conducted with pregnant rabbits dosed with mometasone furoate by either the topical dermal route or oral route throughout the period of organogenesis. In the study using the topical dermal route, mometasone furoate caused multiple malformations in fetuses (e.g., flexed front paws, gallbladder agenesis, umbilical hernia, hydrocephaly) at doses approximately 12 times the MRHDID (on a mcg/m2 basis with maternal topical dermal doses of 150 mcg/kg and above). In the study using the oral route, mometasone furoate caused increased fetal resorptions and cleft palate and/or head malformations (hydrocephaly and domed head) at a dose approximately 60 times the MRHDID (on a mcg/m2 basis with a maternal oral dose of 700 mcg/kg). At approximately 220 times the MRHDID (on a mcg/m2 basis with a maternal oral dose of 2800 mcg/kg), most litters were aborted or resorbed. No effects were observed at a dose approximately 12 times the MRHDID (on a mcg/m2 basis with a maternal oral dose of 140 mcg/kg).
8.2 Lactation
Risk Summary
There are no available data on the presence of olopatadine or mometasone furoate or its metabolites in human milk, the effects on the breastfed child, or the effects on milk production. Other corticosteroids similar to mometasone furoate, are excreted in human milk. However, mometasone furoate concentrations in plasma after nasal therapeutic doses are low and therefore concentrations in human breast milk are likely to be correspondingly low.
Olopatadine has been identified in the milk of nursing rats following oral administration. It is not known whether topical nasal administration could result in sufficient systemic absorption to produce detectable quantities in human breast milk.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for RYALTRIS and any potential adverse effects on the breast fed infant from RYALTRIS or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of RYALTRIS for the treatment of symptoms associated with seasonal allergic rhinitis have been established in pediatric patients 12 years and older. Use of RYALTRIS for this indication is supported by evidence from adequate and well-controlled studies in adult and pediatric patients 12 years and older [see Clinical Studies (14)].
The safety and effectiveness of RYALTRIS in pediatric patients below the age of 12 years have not been established.
Effect on Growth
Controlled clinical studies have shown that nasal corticosteroids may cause a reduction in growth velocity in pediatric patients. This effect has been observed in the absence of laboratory evidence of HPA axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA axis function. The long-term effects of this reduction in growth velocity associated with nasal corticosteroids, including the impact on final adult height, are unknown. The potential for “catch up” growth following discontinuation of treatment with nasal corticosteroids has not been adequately studied.
The growth of pediatric patients receiving nasal corticosteroids, including RYALTRIS, should be monitored routinely (e.g., via stadiometry). The potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the risk/benefits of non-corticosteroid treatment alternatives.
The potential of mometasone furoate nasal spray 50 mcg to cause growth suppression in susceptible patients or when given at higher doses cannot be ruled out.
8.5 Geriatric Use
There were 20 patients 65 years of age and older treated with RYALTRIS in the clinical studies for seasonal allergic rhinitis [see Clinical Studies (14)]. Of the RYALTRIS-treated patients in these studies, 16 (2.7%) were between 65 to 75 years of age, while 4 (0.7%) were 75 years of age and older.
Clinical studies of RYALTRIS did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
8.6 Hepatic Impairment
No studies have been conducted with RYALTRIS in patients with hepatic impairment. However, there have been reports of concentrations of mometasone furoate appearing to increase with severity of hepatic impairment [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
RYALTRIS contains both olopatadine hydrochloride and mometasone furoate; therefore, the risks associated with overdosage for the individual components described below apply to RYALTRIS.
Olopatadine Hydrochloride: Symptoms of antihistamine overdose may include drowsiness in adults and children. Agitation and restlessness, followed by drowsiness in children. Should overdose occur, symptomatic or supportive treatment is recommended.
Mometasone Furoate: Chronic overdosage with any corticosteroid may result in signs or symptoms of hypercorticism [see Warnings and Precautions (5.6)].
-
11 DESCRIPTION
RYALTRIS is a metered-dose manual nasal spray unit containing an aqueous suspension of a fixed‑dose combination of a histamine-1 (H1) receptor inhibitor (olopatadine hydrochloride) and a corticosteroid (mometasone furoate monohydrate).
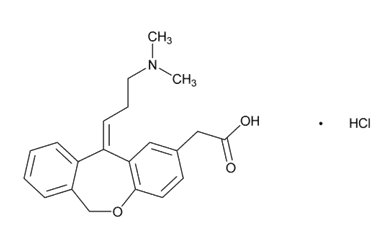
Olopatadine hydrochloride is a white, sparingly water‑soluble crystalline powder. The chemical name for olopatadine hydrochloride is 2‑[(11Z)-11-[3-(dimethylamino)propylidene]-6H-benzo[c][1]benzoxepin-2-yl]acetic acid hydrochloride. It has a molecular weight of 373.88, and its molecular formula is C21H23NO3HCl with the following chemical structure:
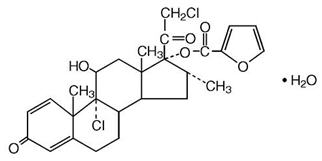
Mometasone furoate monohydrate is an anti-inflammatory corticosteroid having the chemical name [(8S,9R,10S,11S,13S,14S,16R,17R)-9-chloro-17-(2-chloroacetyl)-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] furan-2-carboxylate;hydrate and the following chemical structure:
Mometasone furoate monohydrate is a white powder, with an empirical formula of C27H30Cl2O6H2O and a molecular weight of 539.45. It is practically insoluble in water; slightly soluble in methanol, ethanol, and isopropanol; soluble in acetone and chloroform; and freely soluble in tetrahydrofuran. Its partition coefficient between octanol and water is >5000.
RYALTRIS is a nasal spray containing an isotonic aqueous suspension of olopatadine hydrochloride (equivalent to 0.6% w/v olopatadine base) and mometasone furoate monohydrate (equivalent to 0.025% w/w mometasone furoate on the anhydrous basis). After initial priming (6 sprays), each metered spray delivers 100 microliters of suspension containing 665 mcg of olopatadine hydrochloride (equivalent to 600 mcg olopatadine base) and 25 mcg of mometasone furoate. RYALTRIS also contains benzalkonium chloride, carboxymethyl cellulose sodium, dibasic sodium phosphate heptahydrate, edetate disodium, hydrochloric acid, microcrystalline cellulose, polysorbate 80, sodium chloride, sodium hydroxide, and water. It has a pH of approximately 3.7 [see How Supplied/Storage and Handling (16)].
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
RYALTRIS contains both olopatadine hydrochloride and mometasone furoate. The mechanisms of action described below for the individual components apply to RYALTRIS.
Olopatadine Hydrochloride
Olopatadine is a histamine-1 (H1) receptor inhibitor. The antihistaminic activity of olopatadine has been documented in isolated tissues, animal models, and humans.
Mometasone Furoate
Mometasone furoate is a corticosteroid demonstrating potent anti-inflammatory activity. The precise mechanism of corticosteroid action on allergic rhinitis is not known. Corticosteroids have been shown to have a wide range of inhibitory effects on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in inflammation.
12.2 Pharmacodynamics
Cardiac Electrophysiology
A study specifically designed to evaluate the effect of RYALTRIS on the QT interval has not been conducted.
In a 12-month study, 429 patients treated with olopatadine hydrochloride two sprays per nostril (665 mcg per spray) twice daily, no evidence of any effect of olopatadine hydrochloride on QT prolongation has been observed.
HPA Axis Effect
A study specifically designed to evaluate the effect of RYALTRIS on the HPA axis has not been conducted.
In one study, daily nasal doses of 200 and 400 mcg of mometasone furoate and oral dose of 10 mg prednisone were compared to placebo in 64 patients (22 to 44 years of age) with allergic rhinitis. Adrenal function before and after 36 consecutive days of treatment was assessed by measuring plasma cortisol levels following a 6-hour cosyntropin infusion and by measuring 24-hour urinary free cortisol levels. Mometasone furoate at both the 200- and 400-mcg dose, was not associated with a statistically significant decrease in mean plasma cortisol levels post-cosyntropin infusion or a statistically significant decrease in the 24-hour urinary free cortisol levels compared to placebo. A statistically significant decrease in the mean plasma cortisol levels post-cosyntropin infusion and 24-hour urinary free cortisol levels was detected in the prednisone treatment group compared to placebo.
12.3 Pharmacokinetics
Absorption
After repeated nasal administration of 2 sprays per nostril of RYALTRIS (2660 mcg of olopatadine hydrochloride and 100 mcg of mometasone furoate) twice daily in patients with seasonal allergic rhinitis, the mean (± standard deviation) peak plasma exposure (Cmax) was 19.80 ± 7.01 ng/mL for olopatadine and 9.92 ± 3.74 pg/mL for mometasone furoate, and the mean exposure over the dosing regimen (AUCtau) was 88.77 ± 23.87 ng/mL*hr for olopatadine and 58.40 ± 27.00 pg/mL*hr for mometasone furoate. The median time to peak exposure from a single dose was 1 hour for both olopatadine and mometasone furoate.
Distribution
The protein binding of olopatadine was moderate at approximately 55% in human serum and independent of drug concentration over the range of 0.1 to 1000 ng/mL. Olopatadine binds predominately to human serum albumin.
The in vitro protein binding for mometasone furoate was reported to be 98% to 99% in concentration range of 5 to 500 ng/mL.
Elimination
Following single‑dose nasal administration of a combination of olopatadine and mometasone furoate, the mean elimination half-lives of olopatadine and mometasone furoate were 9 and 18 hours, respectively.
Olopatadine is mainly eliminated through urinary excretion. Approximately 70% of a [14C] olopatadine hydrochloride oral dose was recovered in urine with 17% in the feces. Of the drug‑related material recovered within the first 24 hours in the urine, 86% was unchanged olopatadine, with the balance comprised of olopatadine N-oxide and N-desmethyl olopatadine.
Any absorbed drug is excreted as metabolites mostly via the bile, and to a limited extent, into the urine.
Metabolism
Olopatadine is not extensively metabolized. Based on plasma metabolite profiles following oral administration of [14C] olopatadine, at least 6 minor metabolites circulate in human plasma. Olopatadine accounts for 77% of peak plasma total radioactivity and all metabolites amounted to <6% combined. Two of these have been identified as the olopatadine N-oxide and N-desmethyl olopatadine. In in vitro studies with cDNA-expressed human CYP isoenzymes and flavin-containing monooxygenases (FMO), N-desmethyl olopatadine (Ml) formation was catalyzed mainly by CYP3A4, while olopatadine N-oxide (M3) was primarily catalyzed by FMO1 and FMO3. Olopatadine at concentrations up to 33900 ng/mL did not inhibit the in vitro metabolism of specific substrates for CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4. The potential for olopatadine and its metabolites to act as inducers of CYP enzymes has not been evaluated.
Studies have shown that any portion of a mometasone furoate dose that is swallowed and absorbed undergoes extensive metabolism to multiple metabolites. There are no major metabolites detectable in plasma. Upon in vitro incubation, one of the minor metabolites formed is 6ß-hydroxy-mometasone furoate. In human liver microsomes, the formation of the metabolite is regulated by CYP3A4.
Specific Populations
No pharmacokinetic studies were performed in specific populations with RYALTRIS. The pharmacokinetics of the combination of olopatadine and mometasone furoate is expected to reflect that of the individual components, as the pharmacokinetics of the combination was found to be comparable to the individual components.
Patients with Hepatic Impairment: No specific pharmacokinetic study examining the effect of hepatic impairment was conducted. Metabolism of olopatadine is a minor route of elimination.
Administration of a single inhaled dose of 400 mcg mometasone furoate to subjects with mild (n=4), moderate (n=4), and severe (n=4) hepatic impairment resulted in only 1 or 2 subjects in each group having detectable peak plasma concentrations of mometasone furoate (ranging from 50 to 105 pcg/mL). The observed peak plasma concentrations appeared to increase with severity of hepatic impairment; however, the numbers of detectable levels were few.
Patients with Renal Impairment: The mean Cmax values for olopatadine following single nasal doses were not markedly different between healthy subjects (18.1 ng/mL) and patients with mild, moderate, and severe renal impairment (ranging from 15.5 to 21.6 ng/mL). Mean plasma AUC0-12 was 2‑fold higher in patients with severe impairment (creatinine clearance <30 mL/min/1.73 m2). In these patients, peak steady-state plasma concentrations of olopatadine were approximately 10‑fold lower than those observed after higher, 20mg oral doses, twice daily, which were well tolerated.
The effects of renal impairment on mometasone furoate pharmacokinetics have not been adequately investigated.
Pediatric Patients: RYALTRIS pharmacokinetics has not been investigated in patients under 12 years of age [see Use in Specific Populations (8.4)]. Based on population pharmacokinetic analysis among patients 12 years of age and older, the pharmacokinetics of olopatadine and mometasone furoate with RYALTRIS was not influenced by age.
Male and Female Patients: Based on population pharmacokinetic analysis, the pharmacokinetics of olopatadine and mometasone furoate with RYALTRIS was not influenced by gender.
Racial or Ethnic Groups: Based on population pharmacokinetic analysis, the pharmacokinetics of olopatadine and mometasone furoate with RYALTRIS was not influenced by race.
Drug Interaction Studies
There were no clinically relevant differences in the pharmacokinetics of either olopatadine or mometasone furoate when administered in combination compared with administration alone.
Olopatadine: Drug interactions with inhibitors of liver enzymes are not anticipated because olopatadine is eliminated predominantly by renal excretion. Olopatadine did not inhibit the in vitro metabolism of specific substrates for CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4. Based on these data, drug interactions involving P450 inhibition are not expected. Due to the modest protein binding of olopatadine (55%), drug interactions through displacement from plasma proteins are also not expected.
Mometasone Furoate: Inhibitors of CYP3A4: In a drug interaction study, an inhaled dose of mometasone furoate 400 mcg was given to 24 healthy subjects twice daily for 9 days, and ketoconazole 200 mg (as well as placebo) were given twice daily concomitantly on Days 4 to 9. Mometasone furoate plasma concentrations were <150 pcg/mL on Day 3 prior to coadministration of ketoconazole or placebo. Following concomitant administration of ketoconazole, 4 out of 12 subjects in the ketoconazole treatment group (n=12) had peak plasma concentrations of mometasone furoate >200 pcg/mL on Day 9 (211-324 pcg/mL).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies of carcinogenicity, mutagenicity, or impairment of fertility were conducted with RYALTRIS; however, studies are available for the individual active components, olopatadine hydrochloride and mometasone furoate, as described below.
Olopatadine Hydrochloride: Olopatadine demonstrated no tumorigenic potential in mice at oral doses up to 500 mg/kg/day (approximately 510 times the MRHDID on a mg/m2 basis) for 78 weeks or in rats at oral doses up to 200 mg/kg/day (approximately 410 times the MRHDID on a mg/m2 basis) for 104 weeks.
No mutagenic potential was observed when olopatadine was tested in an in vitro bacterial reverse mutation (Ames) test, an in vitro mammalian chromosome aberration assay, or an in vivo mouse micronucleus test.
Olopatadine administered at an oral dose of 400 mg/kg/day, (approximately 810 times the MRHDID for adults on a mg/m2 basis) produced toxicity in male and female rats, and resulted in a decrease in the fertility index and reduced implantation rate. No effects on reproductive function were observed at 50 mg/kg/day (approximately 100 times the MRHDID on a mg/m2 basis).
Mometasone Furoate: In a 2-year carcinogenicity study in Sprague Dawley rats, mometasone furoate demonstrated no statistically significant increase in the incidence of tumors at inhalation doses up to 67 mcg/kg (approximately 2 times the MRHDID on a mcg/m2 basis). In a 19‑month carcinogenicity study in Swiss CD-1 mice, mometasone furoate demonstrated no statistically significant increase in the incidence of tumors at inhalation doses up to 160 mcg/kg (approximately 4 times the MRHDID on a mcg/m2 basis).
Mometasone furoate increased chromosomal aberrations in an in vitro Chinese hamster ovary‑cell assay, but did not increase chromosomal aberrations in an in vitro Chinese hamster lung cell assay. Mometasone furoate was not mutagenic in the Ames test or mouse lymphoma assay, and was not clastogenic in an in vivo mouse micronucleus assay and a rat bone marrow chromosomal aberration assay, or a mouse male germ-cell chromosomal aberration assay. Mometasone furoate also did not induce unscheduled DNA synthesis in vivo in rat hepatocytes.
In reproductive studies in rats, impairment of fertility was not produced by subcutaneous doses up to 15 mcg/kg (approximately equivalent to the MRHDID on a mcg/m2 basis).
-
14 CLINICAL STUDIES
The efficacy of RYALTRIS was evaluated in two multicenter, randomized, double-blind, placebo-and active-controlled clinical studies of 2-week duration in Study 1 (NCT02631551) and Study 2 (NCT02870205). The two studies were of similar design, including a single blind, placebo run-in period for 7 to 10 days, and enrolled a total of 2352 patients 12 years of age and older with seasonal allergic rhinitis. Patients had a history of seasonal allergic rhinitis for at least 2 years prior to screening, a positive skin prick test (wheal diameter 5mm or greater than negative diluent control) to relevant seasonal allergens (tree/grass pollen in Study 1 and ragweed/mountain cedar pollen in Study 2), and nasal symptoms defined as a 12-hour rTNSS ≥8 out of 12 and a congestion score ≥2 for the morning (AM) assessment at screening.
In Studies 1 and 2, patients were randomized to 1 of 4 treatment groups: RYALTRIS 2 sprays (665 mcg olopatadine hydrochloride and 25 mcg mometasone furoate per spray) per nostril twice daily, olopatadine hydrochloride nasal spray 2 sprays (665 mcg per spray) per nostril twice daily, mometasone furoate nasal spray 2 sprays (25 mcg per spray) per nostril twice daily, and vehicle placebo for 2 weeks. The olopatadine hydrochloride and mometasone furoate comparators used the same device and vehicle as RYALTRIS but were non-US approved drugs. The demographics in Studies 1 and 2 were similar as shown in Table 2.
Table 2: Study 1 and Study 2 - Summary of Demographics Study 1
(N=1180)Study 2
(N=1172)Age
- Mean (SD)
39 (15)
40 (15)
- Min, Max
12, 87
12, 82
Age Group n (%)
- 12-17
115 (10)
94 (8)
Race n (%)
- White
915 (78)
956 (82)
- Asian
20 (2)
22 (2)
- American Indian or Alaska Native
3 (0.3)
3 (0.3)
- Black or African American
230 (20)
181 (15)
- Native Hawaiian or Other Pacific Islander
4 (0.3)
1 (<0.1)
- Other*
8 (0.7)
9 (0.8)
Ethnicity n (%)
- Hispanic or Latino
279 (24)
329 (28)
Gender n (%)
- Female
762 (65)
737 (63)
N = number of subjects in study; n=number of subjects with data available; Min=minimum; Max=maximum; SD=standard deviation. % is based on N (total number of patients in the study)
*Other = Study 1: undefined and Study 2: White and American Indian, Multi-Racial, Mixed, African American and Caucasian, Caucasian and Hispanic, Pakistan and Caucasian.
The primary endpoint for both studies was the change from baseline in average morning (AM) and evening (PM) subject reported 12-hour reflective total nasal symptom score (rTNSS) over the 14-day treatment period. Secondary endpoints included change from baseline in average AM and PM subject-reported 12-hour instantaneous total nasal symptom score (iTNSS) over the 14‑day treatment period and change from baseline in average AM and PM subject-reported 12‑hour reflective total ocular symptom score (rTOSS) over the 14-day treatment period. The rTNSS and iTNSS were calculated as the sum of the patient-reported symptom scores of 4 individual nasal symptoms (rhinorrhea, nasal congestion, sneezing, and nasal itching) on a 0 to 3 categorical severity scale (0=absent, 1=mild, 2=moderate, and 3=severe). Similarly, rTOSS and iTOSS were calculated as the sum of patient’s scoring of 3 individual ocular symptoms (itching/burning, tearing/watering, and redness) on a 0 to 3 categorical severity scale (0=absent, 1=mild, 2=moderate, and 3=severe). Patients were required to record symptom severity daily (morning [AM] and evening [PM]), reflecting over the previous 12 hours (reflective) or at the time of dosing (instantaneous). The primary efficacy endpoint was the mean change from baseline in average AM and PM patient-reported 12-hour rTNSS over the 2-week treatment period. The average AM and PM rTNSS (maximum score of 12) was assessed as the change from baseline for each day and then averaged over a 2-week treatment period.
In both studies, treatment with RYALTRIS resulted in a statistically significant improvement in rTNSS compared to olopatadine hydrochloride and to mometasone furoate as well as to placebo (except for Study 1 comparison to mometasone furoate, 95% CI -0.8-0.0). Results from both studies are shown in Table 3.
Table 3: Mean Change from Baseline in Reflective Total Nasal Symptom Scores Over 2 Weeks* in Adults and Pediatric Patients ≥ 12 Years with Seasonal Allergic Rhinitis in Study 1 and Study 2 Treatment
(2 sprays / nostril twice daily)Study 1 Study 2 N Baseline Mean Change From
Baseline
LS MeanTreatment Effect Difference
LS Mean, (95% CI)N Baseline Mean Change From
Baseline
LS MeanTreatment Effect Difference
LS Mean, (95% CI)RYALTRIS
299
10.1
-3.5
--
291
10.1
‑3.5
--
Olopatadine HCl nasal spray‡
294
10.3
-2.9
-0.6 †
(-1.0, -0.2)290
10.2
‑3.1
‑0.4, †
(‑0.8, ‑0.1)
Mometasone furoate nasal spray‡
294
10.2
-3.1
-0.4
(-0.8, 0.0)293
10.2
‑3.1
‑0.5, †
(‑0.9, ‑0.1)
Placebo
283
10.2
-2.5
-1.0, †
(-1.3, -0.6)290
10.3
‑2.4
‑1.1, †
(‑1.5, ‑0.7)
* Average of AM and PM rTNSS for each day (maximum score = 12) and averaged over the 2-week treatment period.
† Statistically significant difference (p<0.05) using a gatekeeping strategy.
‡ Non-US approved drugs
Least Square (LS) Means, 95% Confidence Intervals (CIs), and p-values were based on the mixed model repeated measures model, adjusting for covariates that included treatment, site, baseline 12-hour reflective total nasal symptom score, and study day as the within-patient effect.
In the two studies, RYALTRIS also demonstrated statistically significant improvement in iTNSS as compared with placebo. Results from both studies are shown in Table 4.
Table 4: Mean Change from Baseline in Instantaneous Total Nasal Symptom Scores Over 2 Weeks* in Adults and Pediatric Patients ≥ 12 Years with Seasonal Allergic Rhinitis in Study 1 and Study 2 Treatment
(2 sprays/nostril twice daily)Study 1
Study 2
N
Baseline Mean
Change From
Baseline
LS MeanTreatment Effect Difference
LS Mean, (95% CI)N
Baseline Mean
Change From
Baseline
LS MeanTreatment Effect Difference
LS Mean, (95% CI)RYALTRIS
299
9.2
-3.0
--
291
9.2
-3.1
--
Olopatadine HCl nasal spray‡
294
9.4
-2.5
-0.5
(-0.9, -0.2)290
9.4
-2.7
-0.4, †
(-0.8, -0.0)Mometasone furoate nasal spray‡
294
9.3
-2.7
-0.4
(-0.7, -0.0)293
9.4
-2.6
-0.5, †
(-0.9, -0.1)Placebo
283
9.3
-2.1
-0.9, †
(-1.3, -0.6)290
9.6
-2.2
-0.9, †
(-1.3, -0.6)* Average of AM and PM iTNSS for each day (maximum score = 12) and averaged over the 2-week treatment period.
† Statistically significant difference (p<0.05)
‡ Not commercially marketed
Least Square (LS) Means, 95% Confidence Intervals (CIs), and p-values were based on the mixed model repeated measures model, adjusting for covariates that included treatment, site, baseline 12-hour reflective total nasal symptom score, and study day as the within-patient effect.
RYALTRIS demonstrated statistically significant improvement compared with placebo in the change from baseline in average morning and evening patient-reported 12‑hour rTOSS (LS mean difference from placebo for Study 1: -0.5, 95% CI: -0.8, -0.2); for Study 2: ‑0.5, 95% CI: -0.8, -0.2) and iTOSS (LS mean difference for Study 1: -0.5, 95% CI: -0.8, -0.2); for Study 2: ‑0.5, 95% CI: -0.8, -0.2) over a 2‑week treatment period.
Onset of action, defined as the first time point after initiation of treatment when RYALTRIS demonstrated a statistically significant change from baseline in iTNSS compared with placebo, was assessed in both studies. Onset of action was observed within 15 minutes following the initial dose of RYALTRIS. Following the initial dose, iTNSS improved over the first week and was sustained through 2 weeks of treatment (Study 1).
The subjective impact of seasonal allergic rhinitis on a patient’s health-related quality of life was evaluated by the Rhinoconjunctivitis Quality of Life Questionnaire - Standardized Activities (RQLQ[S]) (28 questions in 7 domains [activities, sleep, non-nose/eye symptoms, practical problems, nasal symptoms, eye symptoms, and emotional] evaluated on a 7-point scale, in which 0=no impairment and 6=maximum impairment). An overall RQLQ(S) score is calculated from the mean of all items in the instrument. A change from baseline of at least 0.5 points is considered a clinically meaningful improvement. In each of these studies, treatment with RYALTRIS resulted in a statistically significant greater decrease from baseline in the overall RQLQ(S) than placebo (LS mean difference from placebo for Study 1: -0.5 [-0.8, -0.3]; for Study 2: ‑0.5 [95% CI: ‑0.7, ‑0.2]). In these studies, the treatment differences between RYALTRIS and the monotherapies were less than the minimum important difference of 0.5 points.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
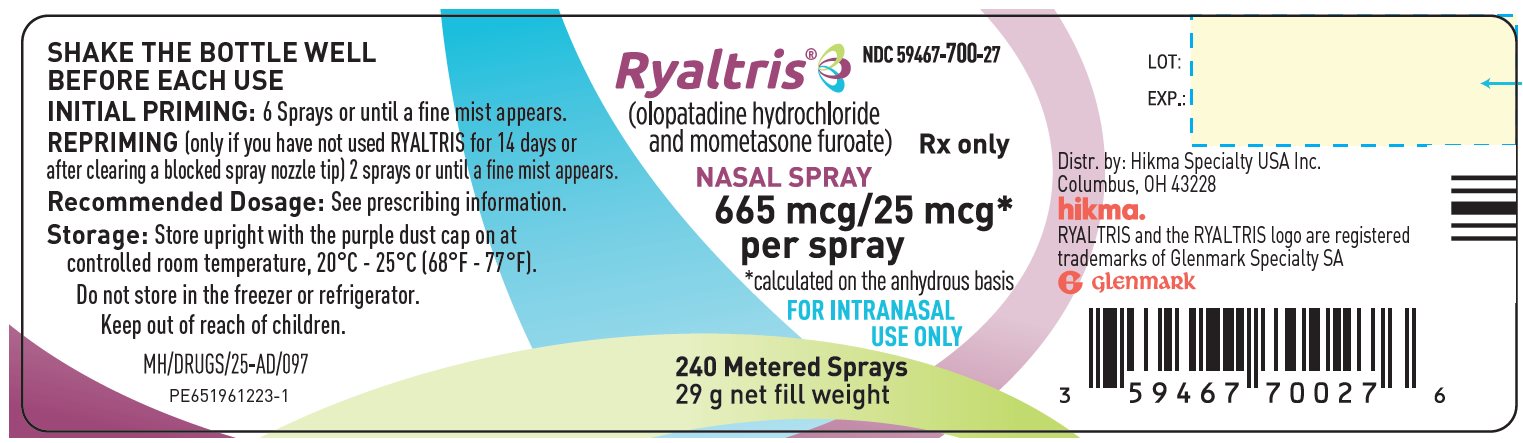
RYALTRIS (NDC: 59467-700-27) is supplied in a white plastic bottle fitted with a metered‑dose spray nozzle unit. Each bottle contains a net fill weight of 31 g and will deliver 240 metered sprays in addition to six (6) initial priming sprays [see Description (11)].
Each spray delivers a volume of 0.1 mL suspension as a fine mist, containing 665 mcg of olopatadine hydrochloride equivalent to 600 mcg of olopatadine (base) and 25 mcg of mometasone furoate monohydrate (on the anhydrous basis). The bottle should be discarded after 240 sprays have been used.
Storage
Store RYALTRIS upright with the purple dust cap on at room temperature (see USP Controlled Room Temperature, between 20°C and 25°C, or between 68°F and 77°F, with excursions permitted between 15°C to 30°C or between 59°F to 86°F). Do not store in a freezer or refrigerator.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Local Nasal Adverse Reactions
Nasal antihistamines are associated with epistaxis, nasal ulceration, and nasal septal perforation. Nasal corticosteroids are associated with epistaxis, nasal septal perforation, Candida albicans infection, and impaired wound healing [see Warnings and Precautions (5.1)].
Somnolence and Impaired Mental Alertness
Patients should be cautioned against engaging in hazardous occupations requiring complete mental alertness and motor coordination such as driving or operating machinery after administration of RYALTRIS [see Warnings and Precautions (5.2)]. Somnolence has been reported in some patients (2 of 789 patients) taking RYALTRIS in controlled clinical studies in seasonal allergic rhinitis [see Adverse Reactions (6.1)].
Concurrent Use of Alcohol and Other Central Nervous System Depressants
Patients should be advised to avoid concurrent use of RYALTRIS with alcohol or other CNS depressants because additional reductions in alertness and additional impairment of CNS performance may occur [see Warnings and Precautions (5.2)].
Administration Information
Patients should be instructed to shake the bottle well before each use and prime RYALTRIS before initial use by releasing 6 sprays or until a fine mist appears. When RYALTRIS has not been used for 14 days or more, patients should re-prime with 2 sprays or until a fine mist appears. Patients should be instructed to avoid spraying RYALTRIS into their eyes [see Dosage and Administration (2)].
Glaucoma and Cataracts
Patients should be informed that nasal and inhaled corticosteroids may result in the development of glaucoma and/or cataracts. Therefore, close monitoring is warranted in patients with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts [see Warnings and Precautions (5.3)].
Hypersensitivity Reactions
Hypersensitivity reactions can occur with RYALTRIS. Hypersensitivity reactions including wheezing, have occurred after the nasal administration of mometasone furoate. Discontinue RYALTRIS if such reactions occur [see Contraindications (4) and Warnings and Precautions (5.4)].
Immunosuppression and Risk of Infections
Persons who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chickenpox or measles, and patients should also be advised that if they are exposed, medical advice should be sought without delay. Inform patients of potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex [see Warnings and Precautions (5.5)].
Potential Drug Interactions
Patients should be advised to be cautious if RYALTRIS is co-administered with ketoconazole or other known strong CYP3A4 inhibitors (e.g., ritonavir, cobicistat-containing products, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, telithromycin) [see Drug Interactions (7.2)].
Distributed by:
Hikma Specialty USA Inc.
Columbus, OH 43228
RYALTRIS and the RYALTRIS logo are registered trademarks of Glenmark Specialty SA
Under license from Glenmark Specialty SA
Revised: April 2025
PN 141197
-
PATIENT PACKAGE INSERT
- PATIENT INFORMATION
RYALTRIS (rye - al’ - tris)
(olopatadine hydrochloride and mometasone furoate monohydrate nasal spray)
Important: For use in your nose only. Do not spray RYALTRIS into your eyes or mouth.
What is RYALTRIS?
RYALTRIS is a prescription nasal spray that contains 2 medicines, olopatadine hydrochloride, an antihistamine, and mometasone furoate, a corticosteroid. RYALTRIS is used to treat symptoms of seasonal allergies in people 12 years of age and older.
It is not known if RYALTRIS is safe and effective in children under 12 years of age.
Do not use RYALTRIS if you are allergic to olopatadine hydrochloride, mometasone furoate monohydrate, or any of the ingredients in RYALTRIS. See the end of this Patient Information leaflet for a complete list of ingredients in RYALTRIS. Ask your healthcare provider if you are not sure.
Before you use RYALTRIS, tell your healthcare provider about all of your medical conditions, including if you:
- have had recent nasal sores, nasal surgery, or nasal injury.
- have eye or vision problems, such as cataracts or glaucoma (increased pressure in your eyes).
- have tuberculosis or any untreated fungal, bacterial, viral infections, or eye infections caused by herpes.
- have been near someone who has chickenpox or measles.
- are not feeling well or have any other symptoms that you do not understand.
- are pregnant or plan to become pregnant. It is not known if RYALTRIS will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if RYALTRIS passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby while using RYALTRIS.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you take:
- certain medicines for HIV (such as ritonavir, atazanavir, indinavir, nelfinavir, and saquinavir)
- cobicistat-containing products
- certain antifungals (such as ketoconazole or itraconazole)
- certain antibiotics (such as clarithromycin and telithromycin)
- certain antidepressants (such as nefazodone)
RYALTRIS and other medicines may affect each other, causing side effects.
Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider or pharmacist when you get a new medicine.
How should I use RYALTRIS?
- Read the Instructions for Use at the end of this Patient Information leaflet for information about the right way to use RYALTRIS.
- RYALTRIS is for use in your nose only. Do not spray it into your eyes or mouth.
- Use RYALTRIS exactly as your healthcare provider tells you to use it.
- If a child accidentally swallows RYALTRIS or you use too much RYALTRIS, call your healthcare provider or go to the nearest hospital emergency room right away.
- See your healthcare provider regularly to check your symptoms while using RYALTRIS and to check for side effects.
What should I avoid while using RYALTRIS?
- RYALTRIS can cause sleepiness or drowsiness. Do not drive, operate machinery, or do anything that needs you to be alert until you know how RYALTRIS affects you.
- Do not drink alcohol or take any other medicines that may cause you to feel sleepy while using RYALTRIS.
What are the possible side effects of RYALTRIS?
RYALTRIS may cause serious side effects, including the following:
-
nose and throat problems. Symptoms of nose and throat problems may include:
- o nosebleeds
- o sores (ulcers) in the nose
- o
hole in the cartilage between your nose (nasal septal perforation). Symptoms of nasal septal perforation may include:
- ▪ crusting in the nose
- ▪ nosebleeds
- ▪ runny nose
- ▪ whistling sound when you breathe
- slow wound healing. You should not use RYALTRIS until your nose has healed if you have a sore in your nose, if you have had surgery on your nose, or if your nose has been injured.
- thrush (Candida), a certain fungal infection in your nose and throat. Tell your healthcare provider if you have any redness or white-colored patches in your nose or mouth.
- eye problems, including glaucoma or cataracts. You should have regular eye exams when using RYALTRIS.
- allergic reactions. Call your healthcare provider or get emergency medical care if you get any of the following signs of a serious allergic reaction:
- wheezing
- rash
- hives
- swelling of your face, mouth, and tongue
- breathing problems
- immune system problems that may increase your risk of infections. Taking medicines that weaken your immune system makes you more likely to get infections. These infections may include tuberculosis (TB), ocular herpes simplex infections, and infections caused by fungi, bacteria, viruses, and parasites. Avoid contact with people who have contagious diseases, such as chickenpox or measles, while using RYALTRIS. If you come in contact with someone who has chicken pox or measles call your healthcare provider right away. Symptoms of infection may include:
- fever
- aches or pains
- chills
- feeling tired
- adrenal insufficiency. Adrenal insufficiency happens when your adrenal glands do not make enough steroid hormones. Symptoms of adrenal insufficiency can include:
- tiredness
- weakness
- nausea
- vomiting
- low blood pressure
- slowed growth in children. A child’s growth should be checked regularly while using RYALTRIS.
- sleepiness or drowsiness.
The most common side effects of RYALTRIS include the following:
- unpleasant taste
- nosebleeds
- nasal discomfort
Tell your healthcare provider if you have any side effects that bother you or that do not go away. These are not all of the possible side effects of RYALTRIS. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088.
How should I store RYALTRIS?
- Store RYALTRIS upright with the purple dust cap on at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze or refrigerate.
- Safely throw away medicine that is out of date or no longer needed.
- Throw away your RYALTRIS bottle after using 240 sprays after the first priming. Even though the bottle may not be completely empty, you may not get the correct dose of medicine if you continue to use it.
Keep RYALTRIS and all medicines out of reach of children.
General information about the safe and effective use of RYALTRIS
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information. Do not use RYALTRIS for a condition for which it was not prescribed. Do not give RYALTRIS to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about RYALTRIS that is written for health professionals.
What are the ingredients in RYALTRIS?
Active ingredients: olopatadine hydrochloride and mometasone furoate monohydrate
Inactive ingredients: benzalkonium chloride, carboxymethyl cellulose sodium, dibasic sodium phosphate heptahydrate, edetate disodium, hydrochloric acid, microcrystalline cellulose, polysorbate 80, sodium chloride, sodium hydroxide, and water.
For more information, go to www.RYALTRIS.com or call Hikma Specialty USA Inc. at 1‑800‑962-8364.
- This Patient Information has been approved by the U.S. Food and Drug Administration Issued: April 2025
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
RYALTRIS (rye - al’ - tris)
(olopatadine hydrochloride and mometasone furoate monohydrate nasal spray)Important: For use in your nose only. Do not spray RYALTRIS into your eyes or mouth.
Read the Instructions for Use before you start to use RYALTRIS and each time you get a refill. There may be new information. This Instructions for Use does not take the place of talking with your healthcare provider about your medical condition or treatment. Before you use RYALTRIS, make sure your healthcare provider shows you the right way to use it.
Shake the bottle for at least 10 seconds before each use.
When RYALTRIS is not in use, the purple dust cap should always be kept tightly placed on the white nozzle tip.
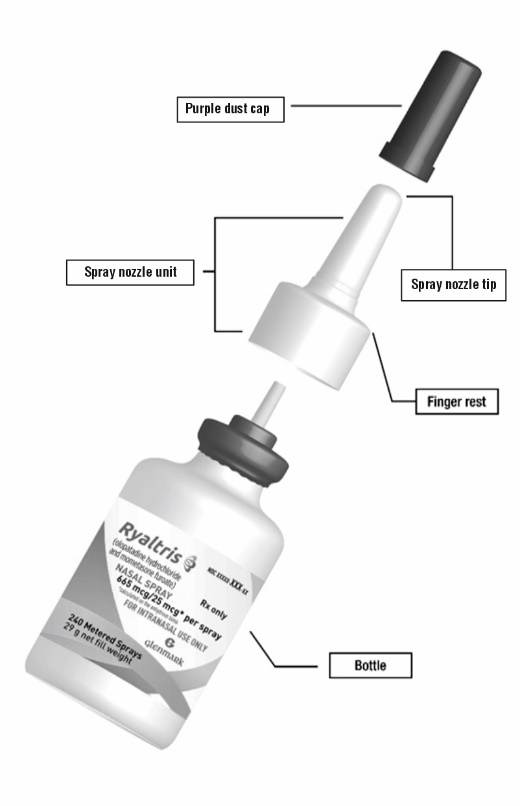
Your RYALTRIS nasal spray bottle (See Figure A)
Figure A
Preparing the nasal spray bottle
Before you prime the bottle, shake the bottle for at least 10 seconds.
Before first use, spray the product 6 times or until a fine mist appears. Spray away from your eyes and face.
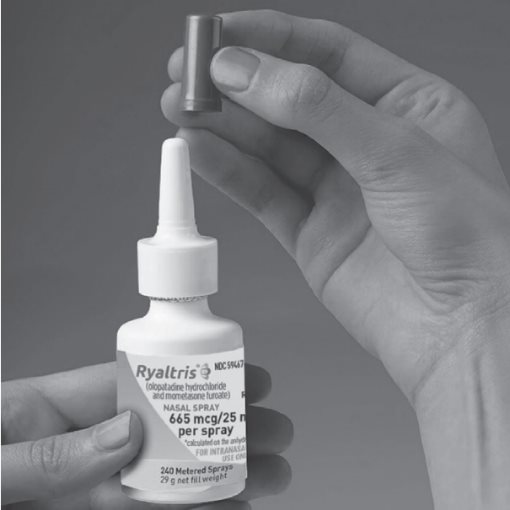
Step 1. Remove the purple dust cap from the spray nozzle tip of the bottle. (See Figure B)
Figure B
Preparing the nasal spray bottle
Step 2. Hold the nasal spray bottle firmly and upright with your index and middle finger on either side of the spray nozzle unit (on finger rests) while supporting the grooved base of the bottle with your thumb.
Step 3. Before first use, push down on the pump quickly and firmly 6 times, releasing the spray into the air, away from the eyes and face until a fine mist appears. (See Figure C)
Figure C
If you do not use RYALTRIS for 14 or more days, you will need to shake the bottle for at least 10 seconds, and prime the pump with 2 sprays or until a fine mist appears.
Your RYALTRIS is now ready for use.
Using your RYALTRIS
Step 4.
Gently blow your nose to clear your nostrils. (See Figure D)
Figure D
Step 5. Shake the bottle for at least 10 seconds before each use (morning and evening).
Step 6. Hold the bottle firmly with your index and middle finger on either side of the spray nozzle unit (on finger rests) while supporting the grooved base of the bottle with your thumb. (See Figure E)
Figure E
Step 7. Hold 1 nostril closed with a finger. Insert the end of the spray nozzle tip into the other nostril, pointing it slightly toward the outside of the nose, away from the nasal septum (the wall between the 2 nostrils). (See Figure F)
Figure F
Step 8. Tilt your head forward slightly. Keep the bottle upright and press down one time quickly and firmly on the finger rests to activate the pump. (See Figure G) Breathe in (inhale) gently through your nose as you spray. Then breathe out through your mouth.
Figure G
- Try not to get any spray into your eyes or directly on your nasal septum (the wall between the 2 nostrils).
Step 9. Repeat Steps 6 through 8 and deliver a second spray in the same nostril.
Step 10. Repeat Steps 6 through 8 with 2 sprays in the other nostril.
- Do not blow your nose for at least 15 minutes after using RYALTRIS, to make sure that you receive all of the medicine.
- Do not tip your head back. This will keep the medicine from going into your throat.
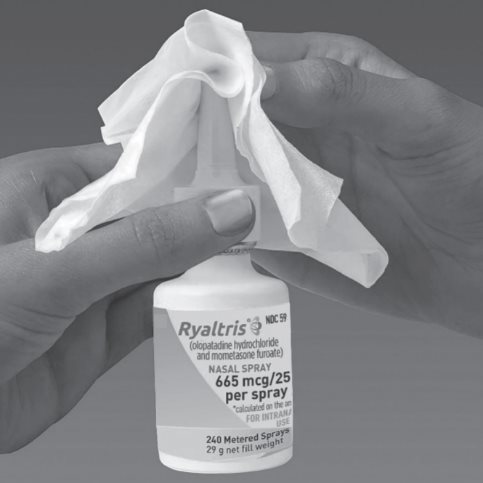
Step 11. To prevent any blockage, wipe the white spray nozzle tip with a clean dry tissue or cloth after each use. (See Figure H)
Figure H
Step 12. Hold the nozzle unit and push the purple dust cap back on the nozzle until you hear a click. (See Figure I)
Figure I
Each bottle of RYALTRIS contains enough medicine for you to spray from the bottle 240 times after the first (initial) priming. You should keep track of the number of sprays used from each bottle of RYALTRIS. Do not count any sprays used for initial priming of the bottle.
How to clear the RYALTRIS nozzle tip if it gets blocked
Do not try to unblock the spray nozzle tip by inserting a pin or other sharp object. (See Figure J) This will damage the spray nozzle tip, and you may not get the correct dose of medicine.
Figure J
Step 13. Remove the spray nozzle unit by gently pulling upward (See Figure K). Remove the purple dust cap and place only the spray nozzle unit in warm water to soak. (See Figure L)
Figure K
Figure L
Step 14. After soaking the spray nozzle tip for 15 minutes, rinse the spray nozzle unit and purple dust cap with warm water and allow them to dry completely. (See Figure M)
Figure M
Step 15. Place the purple dust cap back on the spray nozzle unit and put the spray nozzle unit back on the bottle. (See Figure N)
Figure N
Step 16. After following the steps to clear your blocked spray nozzle tip see “Priming your RYALTRIS pump before use” section above and re-prime using 2 sprays. Replace the purple dust cap, and your RYALTRIS is ready for use.
Repeat the unblocking steps if needed.
How should I store RYALTRIS?
Store RYALTRIS at room temperature between 68°F to 77°F (20°C to 25°C).
Do not freeze or refrigerate.
Do not use RYALTRIS after the expiration date on the label or the box.
Throw away your RYALTRIS bottle after using 240 sprays after first priming. Even though the bottle may not be completely empty, you may not get the correct dose of medicine if you continue to use it.
Keep RYALTRIS and all medicines out of the reach of children.
Distributed by:
Hikma Specialty USA Inc.
Columbus, OH 43228RYALTRIS and the RYALTRIS logo are registered trademarks of Glenmark Specialty SA
Under license from Glenmark Specialty SA
This Instructions for Use has been approved by the U.S. Food and Drug Administration. April 2025
- PRINCIPAL DISPLAY PANELNDC: 59467-700-27RYALTRIS – olopatadine hydrochloride and mometasone furoate240 Metered Sprays Bottle Label
- PRINCIPAL DISPLAY PANELNDC: 59467-700-27 RYALTRIS – olopatadine hydrochloride and mometasone furoate240 Metered Sprays Carton
-
INGREDIENTS AND APPEARANCE
RYALTRIS
olopatadine hydrochloride and mometasone furoate spray, meteredProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59467-700 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLOPATADINE HYDROCHLORIDE (UNII: 2XG66W44KF) (OLOPATADINE - UNII:D27V6190PM) OLOPATADINE HYDROCHLORIDE 665 ug MOMETASONE FUROATE MONOHYDRATE (UNII: MTW0WEG809) (MOMETASONE - UNII:8HR4QJ6DW8) MOMETASONE FUROATE 25 ug Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROCHLORIC ACID (UNII: QTT17582CB) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59467-700-27 12 in 1 BOX 08/15/2022 1 1 in 1 CARTON 1 240 in 1 BOTTLE, SPRAY; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 59467-700-84 12 in 1 BOX 08/15/2022 2 1 in 1 CARTON 2 56 in 1 BOTTLE, SPRAY; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211746 08/15/2022 Labeler - HIKMA SPECIALTY USA INC. (078883518) Registrant - Glenmark Specialty SA (130597813) Establishment Name Address ID/FEI Business Operations Glenmark Pharmaceuticals Limited 650565703 MANUFACTURE(59467-700) , ANALYSIS(59467-700)
Trademark Results [Ryaltris]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RYALTRIS 88605481 not registered Live/Pending |
Glenmark Specialty S.A. 2019-09-05 |
 RYALTRIS 86909378 not registered Dead/Abandoned |
Glenmark Specialty S.A. 2016-02-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.