SYLATRON- peginterferon alfa-2b kit
SYLATRON by
Drug Labeling and Warnings
SYLATRON by is a Prescription medication manufactured, distributed, or labeled by Merck Sharp & Dohme Corp.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SYLATRON safely and effectively. See full prescribing information for SYLATRON.
SYLATRON™ (peginterferon alfa-2b)
for injection, for subcutaneous use
Initial U.S. Approval: 2011WARNING: DEPRESSION AND OTHER NEUROPSYCHIATRIC DISORDERS
See full prescribing information for complete boxed warning.
The risk of serious depression, with suicidal ideation and completed suicides, and other serious neuropsychiatric disorders are increased with alpha interferons, including SYLATRON. Permanently discontinue SYLATRON in patients with persistently severe or worsening signs or symptoms of depression, psychosis, or encephalopathy. These disorders may not resolve after stopping SYLATRON [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
INDICATIONS AND USAGE
SYLATRON is an alpha interferon indicated for the adjuvant treatment of melanoma with microscopic or gross nodal involvement within 84 days of definitive surgical resection including complete lymphadenectomy. (1)
DOSAGE AND ADMINISTRATION
- 6 mcg/kg/week subcutaneously for 8 doses followed by;
- 3 mcg/kg/week subcutaneously for up to 5 years. (2.1)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Depression and other serious neuropsychiatric adverse reactions. (5.1)
- History of significant or unstable cardiac disease. (5.2)
- Retinal disorders. (5.3)
- Child-Pugh score >6 (class B and C). (4, 5.4)
- Hypothyroidism, hyperthyroidism, hyperglycemia, diabetes mellitus that cannot be effectively treated by medication. (4, 5.5)
ADVERSE REACTIONS
Most common adverse reactions (>60%) are: fatigue, increased ALT, increased AST, pyrexia, headache, anorexia, myalgia, nausea, chills, and injection site reaction. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Drugs metabolized by cytochrome P-450 (CYP) enzymes: Monitor for potential increased toxicities of drugs with a narrow therapeutic range metabolized by CYP1A2 or CYP2D6 when coadministered with SYLATRON. (7)
USE IN SPECIFIC POPULATIONS
- Pregnancy: Based on animal data, may cause fetal harm. (8.1)
- Pediatrics: Safety and efficacy in patients <18 years old have not been established. (8.4)
- Renal Impairment: Reduce the dose of SYLATRON by 25% in patients with moderate renal impairment and 50% in patients with severe renal impairment or end-stage renal disease (ESRD) requiring dialysis. (2.1, 8.7)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: DEPRESSION AND OTHER NEUROPSYCHIATRIC DISORDERS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
2.2 Dose Modification Guidelines
2.3 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Depression and Other Serious Neuropsychiatric Adverse Reactions
5.2 Cardiovascular Adverse Reactions
5.3 Retinopathy and Other Serious Ocular Adverse Reactions
5.4 Hepatic Failure
5.5 Endocrinopathies
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: DEPRESSION AND OTHER NEUROPSYCHIATRIC DISORDERS
The risk of serious depression, with suicidal ideation and completed suicides, and other serious neuropsychiatric disorders are increased with alpha interferons, including SYLATRON. Permanently discontinue SYLATRON in patients with persistently severe or worsening signs or symptoms of depression, psychosis, or encephalopathy. These disorders may not resolve after stopping SYLATRON [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
- The recommended starting dose is 6 mcg/kg/week subcutaneously for 8 doses, followed by 3 mcg/kg/week subcutaneously for up to 5 years.
- Premedicate with acetaminophen 500 to 1000 mg orally 30 minutes prior to the first dose of SYLATRON and as needed for subsequent doses.
- The recommended starting doses of SYLATRON in patients with moderate or severe renal impairment or end-stage renal disease (ESRD) are listed in Table 1 [see Use in Specific Populations (8.7)]. No dose adjustment is needed for patients with a creatinine clearance (CLcr) > 50 mL/min/1.73m2.
Table 1: Recommended Starting Dose for Moderate and Severe Renal Impairment and End-Stage Renal Disease Degree of Renal Impairment Creatinine Clearance (mL/min/1.73m2) Initial doses for 8 weeks Follow-up doses for 5 years Moderate 30 – 50 4.5 mcg/kg/week 2.25 mcg/kg/week Severe <30 3 mcg/kg/week 1.5 mcg/kg/week End-Stage Renal Disease On dialysis 3 mcg/kg/week 1.5 mcg/kg/week 2.2 Dose Modification Guidelines
Guidelines for Dose Modification provided below are based on the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE Version 2.0).
- Permanently discontinue SYLATRON for:
- Persistent or worsening severe neuropsychiatric disorders
- Grade 4 non-hematologic toxicity
- Inability to tolerate a dose of 1 mcg/kg/wk
- New or worsening retinopathy
- Withhold SYLATRON dose for any of the following:
- Absolute Neutrophil Count (ANC) less than 0.5×109/L
- Platelet Count (PLT) less than 50×109/L
- ECOG PS greater than or equal to 2
- Non-hematologic toxicity greater than or equal to Grade 3
- Resume dosing at a reduced dose (see Table 1) when all of the following are present:
- Absolute Neutrophil Count (ANC) greater than or equal to 0.5×109/L
- Platelet Count (PLT) greater than or equal to 50×109/L
- ECOG PS 0-1
- Non-hematologic toxicity has completely resolved or improved to Grade 1
Table 2: SYLATRON Dose Modifications Starting Dose Dose Modifications for Doses 1 to 8 6 mcg/kg/week First Dose Modification: 3 mcg/kg/week Second Dose Modification: 2 mcg/kg/week Third Dose Modification: 1 mcg/kg/week Permanently discontinue if unable to tolerate 1 mcg/kg/week Starting Dose Dose Modifications for Doses 9 to 260 3 mcg/kg/week First Dose Modification: 2 mcg/kg/week Second Dose Modification: 1 mcg/kg/week Permanently discontinue if unable to tolerate 1 mcg/kg/week 2.3 Preparation and Administration
Reconstitute SYLATRON with 0.7 mL of Sterile Water for Injection, USP. The Sterile Water for Injection supplied contains 5 mL. Each vial of Sterile Water for Injection is intended for single dose. Discard any unused Sterile Water for Injection, USP.
Table 3: Reconstitution of SYLATRON Single-Dose Vials SYLATRON Single-Dose Vial Diluent (Sterile Water for Injection, USP) Deliverable Product and Volume Final Concentration - * Total vial content of SYLATRON is 296 mcg.
- † Total vial content of SYLATRON is 444 mcg.
- ‡ Total vial content of SYLATRON is 888 mcg.
200 mcg* add 0.7 mL = 200 mcg in 0.5 mL 40 mcg/0.1 mL 300 mcg† add 0.7 mL = 300 mcg in 0.5 mL 60 mcg/0.1 mL 600 mcg‡ add 0.7 mL = 600 mcg in 0.5 mL 120 mcg/0.1 mL - Swirl gently to dissolve the lyophilized powder. DO NOT SHAKE.
- Visually inspect the solution for particulate matter and discoloration prior to administration. Discard if solution is discolored, cloudy, or if particulates are present.
- Do not withdraw more than 0.5 mL of reconstituted solution from each vial.
- Administer SYLATRON subcutaneously. Rotate injection sites.
- If reconstituted solution is not used immediately, store at 2°-8°C (36°-46°F) for no more than 24 hours. Discard reconstituted solution after 24 hours. DO NOT FREEZE.
- For single-dose only. DISCARD ANY UNUSED PORTION.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Depression and Other Serious Neuropsychiatric Adverse Reactions
Peginterferon alfa-2b can cause life-threatening or fatal neuropsychiatric reactions. These include suicide, suicidal and homicidal ideation, depression, and an increased risk of relapse of recovering drug addicts. In the clinical trial, depression occurred in 59% of SYLATRON-treated patients and 24% of patients in the observation group. Depression was severe or life threatening in 7% of SYLATRON-treated patients compared with <1% of patients in the observation arm.
In post-marketing experience, neuropsychiatric adverse reactions have been reported up to 6 months after discontinuation of peginterferon alfa-2b. Based on post-marketing experience with peginterferon alfa-2b and interferon alfa-2b, treatment may also result in aggressive behavior, psychoses, hallucinations, bipolar disorders, mania, and encephalopathy.
Advise patients and their caregivers to immediately report any symptoms of depression or suicidal ideation to their healthcare provider. Monitor and evaluate patients for signs and symptoms of depression and other psychiatric symptoms every 3 weeks during the first 8 weeks of treatment and every 6 months thereafter. Monitor patients during treatment and for at least 6 months after the last dose of SYLATRON. Permanently discontinue SYLATRON for suicidal or homicidal ideation, aggressive behavior towards others, or other severe or persistent psychiatric symptoms; institute psychiatric intervention and follow-up as appropriate.
5.2 Cardiovascular Adverse Reactions
In the clinical trial, cardiac adverse reactions, including myocardial infarction, bundle-branch block, ventricular tachycardia, and supraventricular arrhythmia occurred in 4% of SYLATRON-treated patients compared with 2% of patients in the observation group. In post-marketing experience, hypotension, cardiomyopathy, and angina pectoris have occurred in patients treated with peginterferon alfa-2b.
Permanently discontinue SYLATRON for new onset of ventricular arrhythmia or cardiovascular decompensation.
5.3 Retinopathy and Other Serious Ocular Adverse Reactions
Peginterferon alfa-2b can cause decrease in visual acuity or blindness due to retinopathy. Retinal and ocular changes include macular edema, retinal artery or vein thrombosis, retinal hemorrhages and cotton wool spots, optic neuritis, papilledema, and serous retinal detachment may be induced or aggravated by treatment with peginterferon alfa-2b or other alpha interferons. In the clinical study, two SYLATRON-treated patients developed partial loss of vision due to retinal thrombosis (n=1) or retinopathy (n=1). The overall incidence of serious retinal disorders, visual disturbances, blurred vision, and reduction in visual acuity was <1% in both SYLATRON-treated patients and the observation group.
Perform an eye examination that includes assessment of visual acuity and indirect ophthalmoscopy or fundus photography at baseline in patients with preexisting retinopathy and at any time during SYLATRON treatment in patients who experience changes in vision. Permanently discontinue SYLATRON in patients who develop new or worsening retinopathy.
5.4 Hepatic Failure
Peginterferon alfa-2b, increases the risk of hepatic decompensation and death in patients with cirrhosis. Monitor hepatic function with serum bilirubin, ALT, AST, alkaline phosphatase, and LDH at 2 and 8 weeks, and 2 and 3 months following initiation of SYLATRON, then every 6 months while receiving SYLATRON. Permanently discontinue SYLATRON for evidence of severe (Grade 3) hepatic injury or hepatic decompensation (Child-Pugh score >6 [class B and C]) [see Contraindications (4)].
5.5 Endocrinopathies
Peginterferon alfa-2b can cause new onset or worsening of hypothyroidism, hyperthyroidism, and diabetes mellitus. In the clinical study, 1% of patients developed hypothyroidism; the overall incidence of endocrine disorders was 2% in SYLATRON-treated patients compared to <1% for patients in the observation group.
Obtain TSH levels within 4 weeks prior to initiation of SYLATRON, at 3 and 6 months following initiation, then every 6 months thereafter while receiving SYLATRON. Permanently discontinue SYLATRON in patients who develop hypothyroidism, hyperthyroidism or diabetes mellitus that cannot be effectively managed.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Depression and Other Neuropsychiatric Adverse Reactions [see Warnings and Precautions (5.1)]
- Cardiovascular Adverse Reactions [see Warnings and Precautions (5.2)]
- Retinopathy and Other Serious Ocular Adverse Reactions [see Warnings and Precautions (5.3)]
- Hepatic Failure [see Warnings and Precautions (5.4)]
- Endocrinopathies [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data described below reflect exposure to SYLATRON in 608 patients with surgically resected, AJCC Stage III melanoma. SYLATRON was studied in an open label, multicenter, randomized, observation controlled trial. The median age of the population was 50 years with 10% of patients 65 years or older, and 42% were female. Fourteen percent of patients completed the 5 year treatment schedule.
Patients randomized to SYLATRON were to receive total doses of 48 mcg/kg (6 mcg/kg subcutaneous once weekly for 8 doses), and 780 mcg/kg (3 mcg/kg subcutaneous once weekly until disease recurrence or for up to 5 years), as tolerated. The median total dose received was 42 mcg/kg (range: 6 to 78 mcg/kg) for the first 8 doses, and 136 mcg/kg (range: 1 to 774 mcg/kg) for doses 9 to 260.
Serious adverse events were reported in 199 (33%) patients who received SYLATRON and 94 (15%) patients in the observation group.
The most common adverse reactions experienced by SYLATRON-treated patients were fatigue (94%), increased ALT (77%), increased AST (77%), pyrexia (75%), headache (70%), anorexia (69%), myalgia (68%), nausea (64%), chills (63%), and injection site reaction (62%). The most common serious adverse reactions were fatigue (7%), increased ALT (3%), increased AST (3%), and pyrexia (3%) in the SYLATRON-treated group vs. <1% in the observation group for these reactions.
Thirty three percent of patients receiving SYLATRON discontinued treatment due to adverse reactions. The most common adverse reactions present at the time of treatment discontinuation were fatigue (27%), depression (17%), anorexia (15%), increased ALT (14%), increased AST (14%), myalgia (13%), nausea (13%), headache (13%), and pyrexia (11%). Adverse events that occurred in the clinical study at ≥ 5% incidence in the SYLATRON-treated group and with a greater incidence in patients receiving SYLATRON as compared to the observation group are presented in Table 4.
Table 4: Incidence of Adverse Reactions(*) Occurring in ≥ 5% of Melanoma Patients Treated with SYLATRON and with a Greater Incidence as Compared to Observation Adverse Reaction SYLATRON
N=608Observation
N=628All
Grades
(%)Grade
3 and 4
(%)All
Grades
(%)Grade
3 and 4
(%)- * Adverse reactions were graded using NCI CTCAE, V.2.0.
Any Adverse Reaction 100 51 82 18 General Disorders and Administrative Site Conditions Fatigue 94 16 41 1 Pyrexia 75 4 9 0 Chills 63 1 6 0 Injection Site Reaction 62 1.8 0 0 Metabolic/Laboratory ALT or AST Increased 77 11 26 1 Blood Alkaline Phosphatase Increased 23 0 11 <1 Weight Decreased 11 <1 1 <1 GGT Increased 8 4 1 <1 Proteinuria 7 0 3 0 Anemia 6 <1 2 <1 Nervous System Disorders Headache 70 4 19 1 Dysgeusia 38 0 1 0 Dizziness 35 2 11 <1 Olfactory Nerve Disorder 23 0 1 0 Paraesthesia 21 <1 14 <1 Metabolism and Nutrition Disorders Anorexia 69 3 13 0 Musculoskeletal and Connective Tissue Disorders Myalgia 68 4 23 <1 Arthralgia 51 3 22 1 Gastrointestinal Disorders Nausea 64 3 11 <1 Diarrhea 37 1 8 <1 Vomiting 26 1 4 0 Psychiatric Disorders Depression 59 7 24 <1 Skin and Subcutaneous Tissue Disorders Exfoliative Rash 36 1 4 0 Alopecia 34 0 1 0 Respiratory, Thoracic and Mediastinal Disorders Dyspnea 6 1 2 1 Cough 5 <1 2 0 6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. In a clinical study conducted in patients with melanoma, the incidence of binding antibodies to peg-interferon alfa-2b was approximately 35% (50/144 patients). Among the patients who tested positive for binding antibodies, one patient developed neutralizing antibodies. The impact of antibody formation on pharmacokinetics, safety and efficacy of peg-interferon alfa-2b could not be assessed based on limited available data.
The incidence of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to SYLATRON with the incidence of antibodies to other products may be misleading.
6.3 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of peginterferon alfa-2b as monotherapy and in combination with ribavirin in chronic hepatitis C (CHC) patients. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders
pure red cell aplasia, thrombotic thrombocytopenic purpura
Cardiac Disorders
pericarditis
Ear and Labyrinth Disorders
hearing loss, vertigo, hearing impairment
Endocrine Disorders
diabetic ketoacidosis
Eye Disorders
Vogt-Koyanagi-Harada syndrome
Gastrointestinal Disorders
aphthous stomatitis, pancreatitis, colitis, tongue pigmentation
Infusion Reactions
angioedema, urticaria, bronchoconstriction
Immune System Disorders
systemic lupus erythematosus, erythema multiforme, thyroiditis, thrombotic thrombocytopenic purpura, idiopathic thrombocytopenic purpura, rheumatoid arthritis, interstitial nephritis, and systemic lupus erythematosus
Infections
sepsis, hepatitis B virus reactivation in HCV/HBV co-infected patients
Metabolism and Nutrition Disorders
hypertriglyceridemia
Musculoskeletal and Connective Tissue Disorders
rhabdomyolysis, myositis
Nervous System Disorders
seizures, memory loss, peripheral neuropathy, paraesthesia, migraine headache
Respiratory, Thoracic and Mediastinal Disorders
dyspnea, pulmonary infiltrates, pneumonia, bronchiolitis obliterans, interstitial pneumonitis, sarcoidosis, pulmonary hypertension, and pulmonary fibrosis
Skin and Subcutaneous Tissue Disorders
Stevens-Johnson syndrome, toxic epidermal necrolysis, psoriasis
Vascular Disorders
hypertension, hypotension, stroke
-
7 DRUG INTERACTIONS
Peginterferon alfa-2b inhibits CYP1A2 and CYP2D6 activity. When caffeine (CYP1A2 substrate) or desipramine (CYP2D6 substrate) was coadministered with peginterferon alfa-2b (3 mcg/kg once weekly for two weeks), the exposure to caffeine increased 36% and the exposure to desipramine increased 30% as compared to when caffeine or desipramine was administered alone. Monitor for potential increased toxicities of drugs with a narrow therapeutic range metabolized by CYP1A2 or CYP2D6 when coadministered with SYLATRON. [See Clinical Pharmacology (12.3).]
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies, SYLATRON can cause embryo-fetal harm when administered to a pregnant woman. Available human data with SYLATRON use in pregnant women are insufficient to identify a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Administration of nonpegylated interferon alfa-2b was abortifacient in rhesus monkeys at doses approximately 13 times higher than the recommended dose of 6 mcg/kg/week (see Data).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage is 2-4% and 15-20%, respectively.
Data
Animal Data
In an embryo-fetal development study, rhesus monkeys received non-pegylated interferon alfa-2b daily by intramuscular injection during the period of organogenesis and beyond (Gestation Day 20-80). Nonpegylated interferon alfa-2b was abortifacient at 15 and 30 million international units (IU)/kg (estimated human equivalent of 5 and 10 million IU/kg, based on body surface area). The estimated Intron A human equivalent dose of 5 to 10 million IU/kg daily is approximately equal to a human equivalent dose of 79 to 158 mcg/kg/week of SYLATRON.
8.2 Lactation
Risk Summary
There are no data on the presence of peginterferon alfa-2b in human or animal milk, or on its effects on the breastfed infant, or milk production. Nonpegylated interferon alfa 2-b is present in human milk at low levels. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for SYLATRON and any potential adverse effects on the breastfed infant from SYLATRON or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating SYLATRON [see Use in Specific Populations (8.1)].
Contraception
Females
SYLATRON may cause embryo-fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise female patients of reproductive potential to use effective contraception during treatment with SYLATRON and for at least 10 days after the final dose.
Infertility
Females
Based on animal studies, SYLATRON may transiently impair fertility. [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness in patients below the age of 18 years have not been established.
8.5 Geriatric Use
Clinical studies of SYLATRON did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
8.6 Hepatic Impairment
SYLATRON has not been studied in patients with melanoma who have hepatic impairment. In patients treated for viral hepatitis, peginterferon alfa-2b treatment is contraindicated in those with moderate or severe hepatic impairment (Child-Pugh scores >6). Discontinue SYLATRON if hepatic decompensation (Child-Pugh scores >6) occurs during treatment. [See Contraindications (4) and Warnings and Precautions (5.4).]
8.7 Renal Impairment
Reduce the dose of SYLATRON by 25% in patients with moderate renal impairment (CLcr 30 to 50 mL/min/1.73m2) and 50% in patients with severe renal impairment (CLcr < 30 mL/min/1.73m2) or ESRD requiring dialysis [see Dosage and Administration (2.1)]. A study in subjects with varying degrees of renal impairment showed that the mean exposure (AUC) to peginterferon alfa-2b increased in subjects with moderate and severe renal impairment or ESRD requiring dialysis, as compared to subjects with normal renal function (CLcr > 80 mL/min/1.73m2) following a single 4.5 mcg/kg dose of peginterferon alfa-2b [see Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
SYLATRON, peginterferon alfa-2b, is a covalent conjugate of recombinant alfa-2b interferon with monomethoxy polyethylene glycol (PEG). The average molecular weight of the PEG portion of the molecule is 12,000 daltons. The average molecular weight of the SYLATRON molecule is approximately 31,000 daltons. The specific activity of pegylated interferon alfa-2b is approximately 0.7 × 108 international units/mg protein.
Interferon alfa-2b is a protein with a molecular weight of 19,271 daltons produced by recombinant DNA techniques. It is obtained from the bacterial fermentation of a strain of Escherichia coli bearing a genetically engineered plasmid containing an interferon gene from human leukocytes.
Each vial contains either 296 mcg, 444 mcg or 888 mcg of peginterferon alfa-2b as a sterile, white to off-white lyophilized powder, and dibasic sodium phosphate anhydrous (1.11 mg), monobasic sodium phosphate dihydrate (1.11 mg), polysorbate 80 (0.074 mg), and sucrose (59.2 mg).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Peginterferon alfa-2b is a pleiotropic cytokine; the mechanism by which it exerts its effects in patients with melanoma is unknown.
12.3 Pharmacokinetics
The pharmacokinetics was studied in 32 patients receiving adjuvant therapy for melanoma with SYLATRON according to the recommended dose and schedule (6 mcg/kg/week for 8 doses, followed by 3 mcg/kg/week thereafter). At a dose of 6 mcg/kg/week once weekly, the geometric mean Cmax was 4.4 ng/mL (CV 51%) and the geometric mean AUCtau was 430 ng∙hr/mL (CV 35%) at week 8. The mean terminal half-life was approximately 51 hours (CV 18%). The mean accumulation from week 1 to week 8 was 1.7. After administration of 3 mcg/kg/week once weekly, the mean geometric Cmax was 2.5 ng/mL (CV 33%) and the geometric mean AUCtau was 228 ng∙hr/mL (CV 24%) at week 4. The mean terminal half-life was approximately 43 hours (CV 19%).
Renal Impairment:
Renal clearance accounts for approximately 30% of total peginterferon alfa-2b clearance. The effect of renal impairment on the pharmacokinetics of peginterferon alfa-2b was studied in 24 subjects with normal or impaired renal function after a single 4.5 mcg/kg dose. Compared to subjects with normal renal function (CLcr > 80 mL/min/1.73 m2), the geometric mean AUClast to peginterferon alfa-2b increased by 1.4-fold in subjects with moderate renal impairment (CLcr 30 to 50 mL/min/1.73m2) and 2.1-fold in subjects with severe renal impairment (CLcr < 30 mL/min/1.73m2) or ESRD requiring dialysis [see Use in Specific Populations (8.7)].
No clinically meaningful amounts of peginterferon alfa-2b were removed during hemodialysis following a single 1 mcg/kg dose in subjects with renal impairment.
Drug Interactions:
Peginterferon alfa-2b inhibits CYP1A2 and CYP2D6 activity. In a drug interaction study, healthy subjects received a dose of 200 mg of caffeine (CYP1A2 substrate), 2 mg of midazolam (CYP3A4 substrate), 500 mg of tolbutamide (CYP2C9 substrate), or 50 mg of desipramine (CYP2D6 substrate) before and after two doses of SYLATRON administered subcutaneously at a dose of 3 mcg/kg. The geometric mean AUClast was increased by 36% for caffeine and 30% for desipramine when coadministered with SYLATRON compared to caffeine or desipramine administered alone. No clinically meaningful changes in CYP2C9 activity and CYP3A4 activity were observed. [See Drug Interactions (7).]
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with SYLATRON. Neither peginterferon alfa-2b nor its components, interferon or methoxypolyethylene glycol, caused damage to DNA when tested in the standard battery of mutagenesis assays, in the presence and absence of metabolic activation.
Irregular menstrual cycles were observed in female cynomolgus monkeys given subcutaneous injections of 4239 mcg/m2 peginterferon alfa-2b alone every other day for 1 month (approximately 72 to 144 times the recommended weekly human dose based upon body surface area). These effects included transiently decreased serum levels of estradiol and progesterone, suggestive of anovulation. Normal menstrual cycles and serum hormone levels resumed in these animals 2 to 3 months following cessation of peginterferon alfa-2b treatment. Every other day dosing with 262 mcg/m2 (approximately 3.5 to 7 times the recommended weekly human dose) had no effects on cycle duration or reproductive hormone status. The effects of SYLATRON on male fertility have not been studied.
-
14 CLINICAL STUDIES
The safety and effectiveness of SYLATRON were evaluated in an open-label, multicenter, randomized (1:1) study conducted in 1256 patients with surgically resected, AJCC Stage III melanoma within 84 days of regional lymph node dissection. Patients were randomized to observation (no therapy) (n=629) or to SYLATRON (n=627) at a dose of 6 mcg/kg by subcutaneous injection once weekly for 8 doses followed by a 3 mcg/kg subcutaneous injection once weekly for a period of up to 5 years total treatment. The dose of SYLATRON was adjusted to maintain an ECOG Performance Status of 0 to 1.
The median age of the population was 50 years with 11% of patients 65 years or older, and 42% were female. Forty percent of the study population had microscopic, nonpalpable nodal involvement and 59% had clinically palpable nodes prior to lymphadenectomy. A total of 54% of subjects had one pathologically positive lymph node, 34% had 2 to 4 positive nodes, and 12% had 5 or more. Most subjects had no second primary lesion (98%). Ulceration of the primary lesion was present in 30% of subjects (52% had no ulceration of the primary lesion, and the status was missing/unknown for 18% of subjects). The most common sites were the trunk (43%) or the leg (32%). Eighty-four percent had an International Prognostic Index (IPI) score of 0 and 16% had an IPI score of 1. The main outcome measure was relapse-free survival (RFS), defined as the time from randomization to the earliest date of any relapse (local, regional, in-transit, or distant), or death from any cause. Secondary outcome measures included overall survival.
Patients in the SYLATRON arm received 6 mcg/kg/week for a median of 8.0 weeks. Less than 1% of patients took longer than 9 weeks to complete the 6 mcg/kg/week dosing regimen. Approximately one-third (36%) of patients required dose reductions and 29% of patients required a dose delay, with an average delay of 1.2 weeks, during the initial 8 weeks of SYLATRON. Ninety-four patients (16%) did not continue on to the 3 mcg/kg/week dosing regimen.
Patients who continued on SYLATRON after the initial 8 doses, received 3 mcg/kg/week for a median duration of treatment of 14.3 months. Approximately half (52%) of the patients underwent dose reductions and 70% required dose delays (average delay 2.2 weeks).
Based on 696 RFS events, determined by the Independent Review Committee, median RFS was 34.8 months (95% CI: 26.1, 47.4) and 25.5 months (95% CI: 19.6, 30.8) in the SYLATRON and observation arms, respectively. The estimated hazard ratio for RFS was 0.82 (95% CI: 0.71, 0.96; unstratified log-rank p =0.011) in favor of SYLATRON. Figure 1 shows the Kaplan-Meier curves of RFS.
FIGURE 1: Kaplan-Meier Curves for Relapse-Free Survival 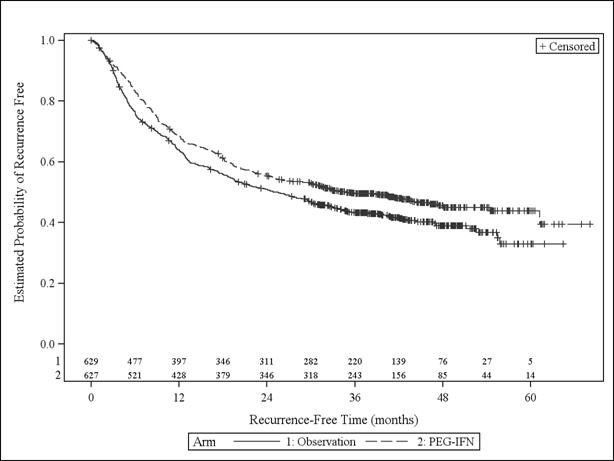
There was no statistically significant difference in survival between the SYLATRON and the observation arms. Based on 525 deaths, the estimated hazard ratio of SYLATRON versus observation was 0.98 (95% CI: 0.82, 1.16).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Each SYLATRON Package Contains: A box containing one 200 mcg per vial of SYLATRON powder and one 5 mL vial of Sterile Water for Injection, USP, 2 B-D Safety Lok syringes with a safety sleeve and 2 alcohol swabs. (NDC: 0085-4347-01) A box containing one 300 mcg per vial of SYLATRON powder and one 5 mL vial of Sterile Water for Injection, USP, 2 B-D Safety Lok syringes with a safety sleeve and 2 alcohol swabs. (NDC: 0085-4348-01) A box containing one 600 mcg per vial of SYLATRON powder and one 5 mL vial of Sterile Water for Injection, USP, 2 B-D Safety Lok syringes with a safety sleeve and 2 alcohol swabs. (NDC: 0085-4349-01) -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
- Advise patients that SYLATRON may be administered with antipyretics at bedtime to minimize common "flu-like" symptoms (including chills, fever, muscle aches, joint pain, headaches, tiredness).
- Advise patients to maintain hydration if experiencing "flu-like" symptoms.
- Advise patients and their caregivers to immediately report any symptoms of depression or suicidal ideation to their healthcare provider during treatment and up to 6 months after the last dose.
- Use SYLATRON during pregnancy only if the potential benefit justifies the potential risk to the fetus [see Use in Specific Populations (8.1, 8.3)].
- Instruct patients to not re-use or share syringes and needles.
- Instruct patients on proper disposal of vials, syringes and needles.
- Advise patients that the Sterile Water for Injection vials supplied contain an excess amount of diluent and only 0.7 mL should be withdrawn to reconstitute SYLATRON. Discard the unused portion of sterile water. Do not save or reuse.
-
SPL UNCLASSIFIED SECTION
Manufactured by:
Merck Sharp & Dohme Corp., a subsidiary of
Merck & Co., Inc., Whitehouse Station, NJ 08889, USA
U.S. License Number 0002For patent information: www.merck.com/product/patent/home.html
BD and Safety-Lok are registered trademarks of Becton, Dickinson and Company.
Copyright © 2011-2019 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.uspi-mk4031-pwi-5ml-1908r008
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 12/2018 MEDICATION GUIDE
SYLATRON™ (SY-LA-TRON)
(Peginterferon alfa-2b)What is the most important information I should know about SYLATRON?
SYLATRON can cause serious mental health problems which can lead to suicide.
SYLATRON may cause you to develop mood or behavior problems that may get worse during treatment with SYLATRON or after your last dose. Call your healthcare provider right away if you, your family, or caregiver notice any of the following:- irritability (getting upset easily)
- depression (feeling low, feeling bad about yourself, or feeling hopeless)
- aggressive behavior, being angry or violent
- thoughts of hurting yourself or others, or suicide
If you have these symptoms, your healthcare provider should carefully monitor you during treatment with SYLATRON and for 6 months after your last dose.
If symptoms get worse or become severe and continue, your healthcare provider may tell you to stop taking SYLATRON permanently. These signs or symptoms may not go away after you stop taking SYLATRON.
See "What are the possible side effects of SYLATRON?" for more information about side effects.What is SYLATRON?
SYLATRON is a prescription medicine that is used to prevent malignant melanoma (a kind of skin cancer) from coming back after it has been removed by surgery. SYLATRON should be started within 84 days of surgery to remove lymph nodes containing cancer.
It is not known if SYLATRON is safe and effective in children less than 18 years of age.Who should not take SYLATRON?
Do not take SYLATRON if you:- have had a serious allergic reaction to peginterferon alfa-2b or to interferon alfa-2b
- have certain types of hepatitis
- have severe liver damage
What should I tell my healthcare provider before taking SYLATRON?
Before you take SYLATRON, tell your healthcare provider about all of your health problems, including if you:- are being treated for a mental illness or had treatment in the past for mental illness, including depression or thoughts of hurting yourself or others or suicide attempts. See "What is the most important information I should know about SYLATRON?"
- have liver damage from drugs or cirrhosis or other liver disease
- have kidney problems or are receiving kidney dialysis treatment
- have ever been addicted to drugs or alcohol
- have or had an overactive or underactive thyroid gland
- have diabetes
- have any other medical problems
- are pregnant or plan to become pregnant. SYLATRON can harm your unborn baby.
- Females who are able to become pregnant should use effective birth control (contraception) during treatment with SYLATRON and for at least 10 days after the final dose of SYLATRON.
- Your healthcare provider should do a pregnancy test before you begin taking SYLATRON. Tell your healthcare provider right away if you become pregnant or you think you might be pregnant during treatment with SYLATRON.
- are breastfeeding or plan to breastfeed. It is not known if SYLATRON passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take SYLATRON.
SYLATRON and certain other medicines may affect each other and cause side effects.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist each time you get a new medicine.
You should not start a new medicine before your talk with the healthcare provider who prescribes you SYLATRON.How should I take SYLATRON? - Take SYLATRON exactly as your healthcare provider tells you to. Your healthcare provider will tell you how much SYLATRON to take and when to take it.
- Do not take more than your prescribed dose. Call your healthcare provider right away if you take too much SYLATRON.
- Inject SYLATRON one time each week unless instructed differently by your healthcare provider. Call your healthcare provider for instructions if you miss a dose.
- SYLATRON is given as an injection under your skin (subcutaneous injection). Your healthcare provider should show you how to prepare and measure your dose of SYLATRON, and how to inject yourself before you use SYLATRON for the first time.
- Expect to get "flu-like" symptoms when taking SYLATRON. To help reduce flu-like symptoms:
- You should take 500 mg to 1,000 mg of acetaminophen 30 minutes before your first dose of SYLATRON.
- Follow your healthcare provider's instructions about taking acetaminophen before future doses of SYLATRON.
- Inject SYLATRON at bedtime to help reduce flu-like symptoms.
- Drink plenty of fluids.
Your healthcare provider will monitor you for side effects during treatment with SYLATRON. If needed, your healthcare provider may:- keep your prescribed dose the same
- reduce your prescribed dose
- tell you to skip a dose or doses, or
- tell you to stop taking SYLATRON permanently
What are the possible side effects of SYLATRON?
SYLATRON can cause serious side effects or worsen existing problems, including:
See "What is the most important information I should know about SYLATRON?"-
Heart problems. Signs and symptoms can include:
- fast heart rate or abnormal heart beat
- trouble breathing or chest pain
-
Serious eye problems. Symptoms can include:
- decrease in vision
- blurred vision
-
Severe or worsening liver problems. Symptoms can include:
- yellowing of your skin or the white part of your eyes
- swelling of your stomach area (abdomen)
-
Thyroid problems. Signs and symptoms can include:
- problems concentrating
- feeling cold or hot all of the time
- weight changes
-
High blood sugar (diabetes). Signs and symptoms can include:
- increased thirst
- urinating more often than normal
- weight loss
- your breath smells like fruit
The most common side effects of SYLATRON include:- flu-like symptoms, which may include fever, headache, tiredness, muscle or joint aches, chills, nausea, or loss of appetite
- feeling sad or depressed
- redness, swelling, or itching around the injection site
- changes in blood tests measuring how your liver works
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1–800–FDA–1088.
You may also report side effects to Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877-888-4231.How should I store SYLATRON? - Store SYLATRON vials in the carton at 59°F to 86°F (15°C to 30°C).
- After mixing, use SYLATRON right away or store it in the refrigerator for no longer than 24 hours at 36°F to 46°F (2°C to 8°C).
- Do not freeze SYLATRON.
General information about the safe and effective use of SYLATRON
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use SYLATRON for a condition for which it was not prescribed. Do not give SYLATRON to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about SYLATRON. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider for information about SYLATRON that is written for healthcare professionals.What are the ingredients in SYLATRON?
Active ingredient: peginterferon alfa-2b
Inactive ingredients: dibasic sodium phosphate anhydrous, monobasic sodium phosphate dihydrate, polysorbate 80, sucrose, sterile water for injection is supplied as a diluent.
Manufactured by:
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ 08889, USA
U.S. License Number 0002
For patent information: www.merck.com/product/patent/home.html
Copyright © 2011-2018 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.
usmg-mk4031-pwi-1812r014
For more information, go to www.SYLATRON.com or call 1-877-888-4231. -
Instructions for Use
SYLATRON™ (SY-LA-TRON)
(peginterferon alfa-2b)
for injection
Powder for InjectionBe sure that you read, understand and follow these instructions before injecting SYLATRON. Your healthcare provider should show you how to prepare, measure, and inject SYLATRON properly before you use it for the first time. Ask your healthcare provider if you have any questions.
Before starting, collect all of the supplies that you will need to use for preparing and injecting SYLATRON. For each injection you will need a SYLATRON vial package that contains:
- 1 vial of SYLATRON powder
- 1 vial of sterile water for injection (diluent). The vial contains an excess amount of sterile water (5 mL). You will only need to withdraw 0.7 mL to prepare your single dose.
- 2 single-dose disposable syringes (BD Safety Lok syringes with a safety sleeve)
- 2 alcohol swabs
You will also need:
- 1 cotton ball or gauze
- 1 sharps disposal container to throw away (dispose of) used syringes, needles, and vials. See "How should I dispose of used syringes, needles, and vials?" at the end of this Instructions for Use.
Important:
- Do not re-use or share syringes and needles.
- The vial of mixed SYLATRON should be used right away. Do not mix more than 1 vial of SYLATRON at a time. If you do not use the vial of the prepared solution right away, store it in a refrigerator and use within 24 hours. See the end of this Instructions for Use for information about "How should I store SYLATRON?"
- Make sure you have the right syringe and needle to use with SYLATRON. Your healthcare provider should tell you what syringes and needles to use to inject SYLATRON.
How should I prepare a dose of SYLATRON?
Before you inject SYLATRON, the powder must be mixed with 0.7 mL of the sterile water for injection (diluent) that comes in the SYLATRON vial package.
- Find a clean, well-lit, flat work surface.
- Get 1 of your SYLATRON vial packages. Check the date printed on the SYLATRON carton. Make sure that the expiration date has not passed. Do not use your SYLATRON vial packages if the expiration date has passed. The medicine in the SYLATRON vial should look like a white to off-white tablet that is whole, or in pieces, or powdered.
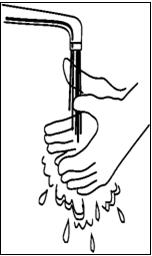
If you have already mixed the SYLATRON solution and stored it in the refrigerator, take it out of the refrigerator before use and allow the solution to come to room temperature. - Wash your hands well with soap and water, rinse and towel dry (See Figure A). Keep your work area, your hands, and injection site clean to decrease the risk of infection.
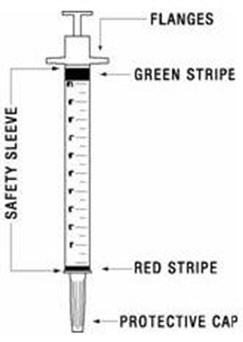
The disposable syringes have needles that are already attached and cannot be removed. Each syringe has a clear plastic safety sleeve that is pulled over the needle for disposal after use. The safety sleeve should remain tight against the flange while using the syringe and moved over the needle only when ready for disposal. (See Figure B)
- Remove the protective wrapper from one of the syringes provided. Use the syringe for steps 4 through 15. Make sure that the syringe safety sleeve is sitting against the flange. (See Figure B)
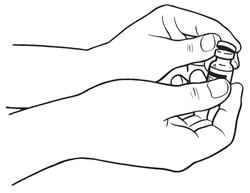
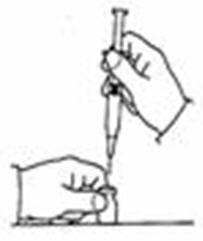
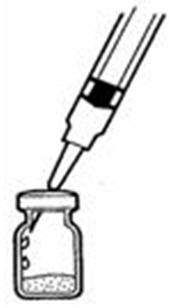
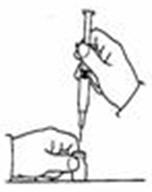
- Remove the protective plastic cap from the tops of both the sterile water for injection (diluent) and the SYLATRON vials (See Figure C). Clean the rubber stopper on the top of both vials with an alcohol swab.
- Carefully remove the protective cap straight off of the needle to avoid damaging the needle point.
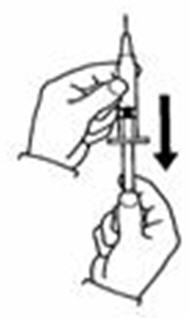
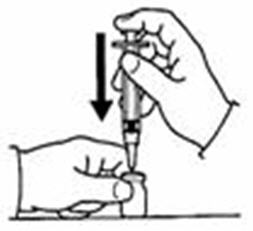
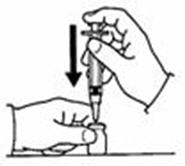
- Fill the syringe with air by pulling back on the plunger to 0.7 mL. (See Figure D)
- Hold the diluent vial upright. Do not touch the cleaned top of the vial with your hands.
- Turn the vial upside down and make sure the tip of the needle is in the liquid.
- Important: The sterile water for injection vial contains an excess amount of sterile water (5 mL). You will only need to withdraw 0.7 mL to prepare your single dose.
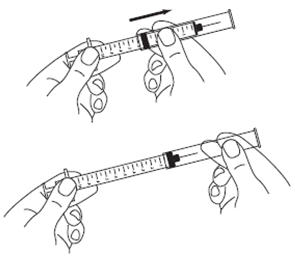
- Withdraw only 0.7 mL of diluent by pulling the plunger back to the 0.7 mL mark on the side of the syringe. (See Figure G)
- With the needle still inserted in the vial, check the syringe for air bubbles.
- If there are any air bubbles, gently tap the syringe with your finger until the air bubbles rise to the top of the syringe.
- Slowly push the plunger up to remove the air bubbles.
- If you push diluent back into the vial, slowly pull back on the plunger to draw the correct amount of diluent back into the syringe.
- Remove the needle from the vial (See Figure H). Do not let the syringe touch anything.
- Throw away the diluent that is left over in the vial. Do not save any leftover diluent or use it again. See "How should I dispose of used syringes, needles, and vials?" at the end of this Instructions for Use.
- Insert the needle through the center of the rubber stopper of the SYLATRON powder vial. Do not touch the cleaned rubber stopper.
- Place the needle tip, at an angle, against the side of the vial. (See Figure I)
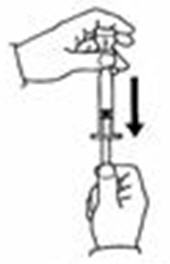
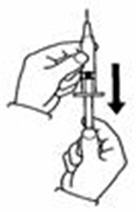
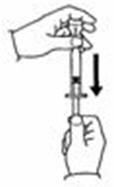
- Slowly push the plunger down to inject the 0.7 mL diluent. The stream of diluent should run down the side of the vial.
- To prevent bubbles from forming, do not aim the stream of diluent directly on the medicine in the bottom of the vial.
- Remove the needle from the vial.
- Firmly grasp the safety sleeve and pull it over the exposed needle until you hear a click (See Figure J). The green stripe on the safety sleeve will completely cover the red stripe on the needle. Dispose of the syringe, needle, and vial in the sharps disposal container. See "How should I dispose of used syringes, needles, and vials?" at the end of this Instructions for Use.
- Firmly grasp the safety sleeve and pull it over the exposed needle until you hear a click (See Figure J). The green stripe on the safety sleeve will completely cover the red stripe on the needle. Dispose of the syringe, needle, and vial in the sharps disposal container. See "How should I dispose of used syringes, needles, and vials?" at the end of this Instructions for Use.
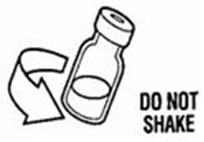
- Gently swirl the vial in a gentle circular motion, until the SYLATRON is completely dissolved (mixed together). (See Figure K)
- Do not shake the vial. If any powder remains undissolved in the vial, gently turn the vial upside down until all of the powder is dissolved.
- The solution may look cloudy or bubbly for a few minutes. If air bubbles form, wait until the solution settles and all bubbles rise to the top.
- After the SYLATRON completely dissolves, the solution should be clear, colorless and without particles. It is normal to see a ring of foam or bubbles on the surface.
- Do not use the mixed solution if you see particles in it, or it is not clear and colorless. Dispose of the syringe and needle in the sharps disposal container. See the section "How should I dispose of used syringes, needles, and vials?" at the end of this Instructions for Use. Then, repeat steps 1 through 17 with a new vial of SYLATRON and diluent to prepare a new syringe.
- After the SYLATRON powder completely dissolves, clean the rubber stopper again with an alcohol swab before you withdraw your dose.
- Unwrap the second syringe provided. You will use it to give yourself the injection.
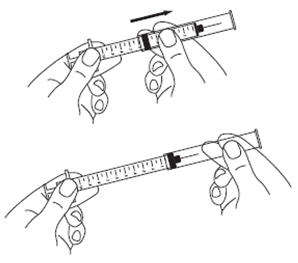
- Carefully remove the protective cap from the needle. Fill the syringe with air by pulling the plunger to the number on the side of the syringe (mL) that matches your prescribed dose. (See Figure L)
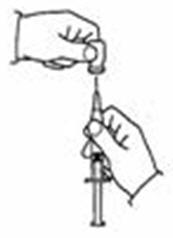
- Hold the SYLATRON vial upright. Do not touch the cleaned top of the vial with your hands. (See Figure M)
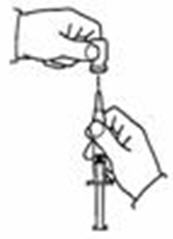
- Insert the needle into the vial containing the SYLATRON solution. Inject the air into the center of the vial. (See Figure N)
- Carefully remove the protective cap from the needle. Fill the syringe with air by pulling the plunger to the number on the side of the syringe (mL) that matches your prescribed dose. (See Figure L)
- Turn the SYLATRON vial upside down. Be sure the tip of the needle is in the SYLATRON solution.
- Hold the vial and syringe with one hand. Be sure the tip of the needle is in the SYLATRON solution. With the other hand, slowly pull the plunger back to fill the syringe with the exact amount of SYLATRON into the syringe your healthcare provider told you to use. (See Figure O)
- Hold the vial and syringe with one hand. Be sure the tip of the needle is in the SYLATRON solution. With the other hand, slowly pull the plunger back to fill the syringe with the exact amount of SYLATRON into the syringe your healthcare provider told you to use. (See Figure O)
- Check for air bubbles in the syringe. If you see any air bubbles, hold the syringe with the needle pointing up. Gently tap the syringe until the air bubbles rise. Then, slowly push the plunger up to remove any air bubbles. If you push solution into the vial, slowly pull back on the plunger again to draw the correct amount of SYLATRON back into the syringe. When you are ready to inject the medicine, remove the needle from the vial. (See Figure P)
How should I choose a site for injection?
The best sites for giving yourself an injection are those areas with a layer of fat between the skin and muscle, like your thigh, the outer surface of your upper arm, and abdomen (See Figure Q). Do not inject yourself in the area near your navel or waistline. If you are very thin, you should only use the thigh or outer surface of the arm for injection.
You should use a different site each time you inject SYLATRON to help avoid soreness at any one site. Do not inject SYLATRON solution into an area where the skin is irritated, red, bruised, infected or has scars, stretch marks, or lumps.
How should I inject a dose of SYLATRON?
- 22.
Clean the skin where the injection is to be given with an alcohol swab. Wait for the area to dry.
- Make sure the safety sleeve of the syringe is pushed firmly against the syringe flange so that the needle is fully exposed. (See Figure B)
- 23.
With one hand, pinch a fold of skin. With your other hand, pick up the syringe and hold it like a pencil.
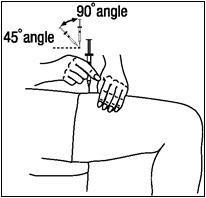
- Insert the needle into the pinched skin at a 45- to 90-degree angle with a quick dart-like motion. (See Figure R)
- After the needle is inserted, remove the hand that you used to pinch your skin. Use it to hold the syringe barrel.
- Pull the plunger of the syringe back very slightly.
- If no blood is present in the syringe, inject the medicine by gently pressing the plunger all the way down the syringe barrel, until the syringe is empty.
-
If blood comes into the syringe, the needle has entered a blood vessel. Do not inject.
- Withdraw the needle and dispose of the syringe and needle in the sharps disposal container. (See the section "How should I dispose of used syringes, needles, and vials?" at the end of this Instructions for Use.)
- If there is bleeding, cover the injection site with a bandage.
- Then, repeat steps 1 through 23 with a new vial of SYLATRON and diluent to prepare a new syringe, and inject the medicine at a new site.
- Insert the needle into the pinched skin at a 45- to 90-degree angle with a quick dart-like motion. (See Figure R)
- 24.
When the syringe is empty, pull the needle out of the skin.
- Place a cotton ball or gauze over the injection site and press for several seconds. Do not massage the injection site.
- If there is bleeding, cover it with a bandage.
- 25.
After injecting your dose:
- Firmly grasp the safety sleeve and pull it over the exposed needle until you hear a click, and the green stripe on the safety sleeve covers the red stripe on the needle. (See Figure S)
- Firmly grasp the safety sleeve and pull it over the exposed needle until you hear a click, and the green stripe on the safety sleeve covers the red stripe on the needle. (See Figure S)
- 26. Dispose of the used syringes, needles, and vials in the sharps disposal container. (See "How should I dispose of used syringes, needles, and vials?" below.)
How should I dispose of used syringes, needles, and vials?
- Put your used syringes and needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose syringes and needles in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used syringes and needles. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Always keep the sharps disposal container out of the reach of children.
- Before mixing, store SYLATRON vials at 59°F to 86°F (15°C to 30°C).
- After mixing, use SYLATRON right away or store it in the refrigerator for up to 24 hours between 36°F to 46°F (2°C to 8°C). Throw away any mixed SYLATRON that is not used within 24 hours.
- Do not freeze SYLATRON.
- Keep SYLATRON away from heat.
Keep SYLATRON and all medicines out of the reach of children.
-
SPL UNCLASSIFIED SECTION
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ 08889, USA
U.S. License Number 0002Revised: August 2019
For patent information: www.merck.com/product/patent/home.html
Copyright © 2011- 2019 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reservedusifu-mk4031-pwi-5ml-1908r002
-
PRINCIPAL DISPLAY PANEL - Kit Carton - 200 mcg
This carton contains:
One single-use vial of SYLATRON
One single-use vial of diluent
Two syringes with 1/2-inch 27-gauge needles
Two alcohol swabsNDC: 0085-4347-01
Rx only
SYLATRON®
(peginterferon alfa-2b)
For InjectionDo not reuse vial
Dispense the enclosed
Medication Guide to each patient.
Dosage and Administration:
See package insert. Read accompanying directions.
Reconstitute as directed in package insert.200 mcg per vial*
For Subcutaneous Use
Delivers 200 mcg in 0.5 mL.
-
PRINCIPAL DISPLAY PANEL - Kit Carton - 300 mcg
This carton contains:
One single-use vial of SYLATRON
One single-use vial of diluent
Two syringes with 1/2-inch 27-gauge needles
Two alcohol swabsNDC: 0085-4348-01
Rx only
SYLATRON®
(peginterferon alfa-2b)
For InjectionDo not reuse vial
Dispense the enclosed
Medication Guide to each patient.
Dosage and Administration:
See package insert. Read accompanying directions.
Reconstitute as directed in package insert.300 mcg per vial*
For Subcutaneous Use
Delivers 300 mcg in 0.5 mL.
-
PRINCIPAL DISPLAY PANEL - Kit Carton - 600 mcg
This carton contains:
One single-use vial of SYLATRON
One single-use vial of diluent
Two syringes with 1/2-inch 27-gauge needles
Two alcohol swabsNDC: 0085-4349-01
Rx only
SYLATRON®
(peginterferon alfa-2b)
For InjectionDo not reuse vial
Dispense the enclosed
Medication Guide to each patient.
Dosage and Administration:
See package insert. Read accompanying directions.
Reconstitute as directed in package insert.600 mcg per vial*
For Subcutaneous Use
Delivers 600 mcg in 0.5 mL.
-
INGREDIENTS AND APPEARANCE
SYLATRON
peginterferon alfa-2b kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0085-4347 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0085-4347-01 1 in 1 CARTON 08/30/2014 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 0.1 mL Part 2 1 VIAL 5 mL Part 1 of 2 SYLATRON
peginterferon alfa-2b injection, powder, lyophilized, for solutionProduct Information Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength peginterferon alfa-2b (UNII: G8RGG88B68) (peginterferon alfa-2b - UNII:G8RGG88B68) peginterferon alfa-2b 40 ug in 0.1 mL Inactive Ingredients Ingredient Name Strength sodium phosphate, dibasic, anhydrous (UNII: 22ADO53M6F) sodium phosphate, monobasic, dihydrate (UNII: 5QWK665956) sucrose (UNII: C151H8M554) polysorbate 80 (UNII: 6OZP39ZG8H) Product Characteristics Color WHITE (White to off-white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103949 03/29/2011 Part 2 of 2 STERILE WATER
inert injection, solutionProduct Information Route of Administration SUBCUTANEOUS Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103949 08/30/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103949 08/30/2014 SYLATRON
peginterferon alfa-2b kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0085-4348 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0085-4348-01 1 in 1 CARTON 03/29/2011 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 0.1 mL Part 2 1 VIAL 5 mL Part 1 of 2 SYLATRON
peginterferon alfa-2b injection, powder, lyophilized, for solutionProduct Information Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength peginterferon alfa-2b (UNII: G8RGG88B68) (peginterferon alfa-2b - UNII:G8RGG88B68) peginterferon alfa-2b 60 ug in 0.1 mL Inactive Ingredients Ingredient Name Strength sodium phosphate, dibasic, anhydrous (UNII: 22ADO53M6F) sodium phosphate, monobasic, dihydrate (UNII: 5QWK665956) sucrose (UNII: C151H8M554) polysorbate 80 (UNII: 6OZP39ZG8H) Product Characteristics Color WHITE (White to off-white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103949 03/29/2011 Part 2 of 2 STERILE WATER
inert injection, solutionProduct Information Route of Administration SUBCUTANEOUS Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103949 08/30/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103949 03/29/2011 SYLATRON
peginterferon alfa-2b kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0085-4349 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0085-4349-01 1 in 1 CARTON 03/29/2011 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 0.1 mL Part 2 1 VIAL 5 mL Part 1 of 2 SYLATRON
peginterferon alfa-2b injection, powder, lyophilized, for solutionProduct Information Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength peginterferon alfa-2b (UNII: G8RGG88B68) (peginterferon alfa-2b - UNII:G8RGG88B68) peginterferon alfa-2b 120 ug in 0.1 mL Inactive Ingredients Ingredient Name Strength sodium phosphate, dibasic, anhydrous (UNII: 22ADO53M6F) sodium phosphate, monobasic, dihydrate (UNII: 5QWK665956) sucrose (UNII: C151H8M554) polysorbate 80 (UNII: 6OZP39ZG8H) Product Characteristics Color WHITE (White to off-white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103949 03/29/2011 Part 2 of 2 STERILE WATER
inert injection, solutionProduct Information Route of Administration SUBCUTANEOUS Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103949 08/30/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103949 03/29/2011 Labeler - Merck Sharp & Dohme Corp. (001317601)
Trademark Results [SYLATRON]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SYLATRON 85195152 4068535 Live/Registered |
MERCK SHARP & DOHME CORP. 2010-12-10 |
 SYLATRON 78529555 not registered Dead/Abandoned |
SCHERING CORPORATION 2004-12-09 |
 SYLATRON 77166324 not registered Dead/Abandoned |
SCHERING CORPORATION 2007-04-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.