MAXI-TUSS DM- chlorpheniramine maleate and dextromethorphan hydrobromide liquid
Maxi-Tuss DM by
Drug Labeling and Warnings
Maxi-Tuss DM by is a Otc medication manufactured, distributed, or labeled by MCR American Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
- cough due to minor throat and bronchial irritation associated with a cold
- alleviates cough to help you sleep
- non narcotic cough suppressant for relief of cough
- itchy, watery eyes
- runny nose
- sneezing
- itching of the nose and throat
-
Warnings
- Do not exceed recommended dosage.
- A persistent cough may be a sign of a serious condition. If cough persists for more than 1 week, tends to recur, or is accompanied by fever, rash, or persistent headache, consult a doctor.
Do not use this product
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product
Do not take this product, unless directed by a doctor if you have
- a persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema, or where cough is accompanied by excessive phlegm
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
-
Directions
Do not exceed recommended dosage.
Adults and children 12 years of age and over: 1 teaspoonful (5 mL) every 4 hours, not to exceed 6 teaspoonfuls in 24 hours, or as directed by a doctor Children 6 to under 12 years of age: ½ teaspoonful (2.5 mL) every 4 hours, not to exceed 3 teaspoonfuls in 24 hours, or as directed by a doctor Children under 6 years of age: Consult a doctor - Other information
- Inactive ingredients
- Questions or Comments?
-
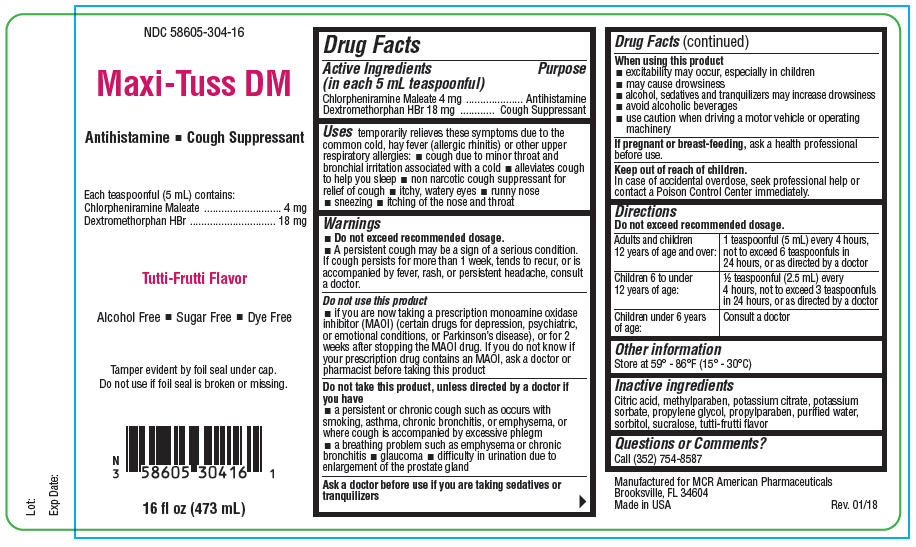
PRINCIPAL DISPLAY PANEL - 473 mL Bottle Label
NDC: 58605-304-16
Maxi-Tuss DM
Antihistamine ◾ Cough Suppressant
Each teaspoonful (5 mL) contains:
Chlorpheniramine Maleate 4 mg
Dextromethorphan HBr 18 mgTutti-Frutti Flavor
Alcohol Free ◾ Sugar Free ◾ Dye Free
Tamper evident by foil seal under cap.
Do not use if foil seal is broken or missing.16 fl oz (473 mL)
-
INGREDIENTS AND APPEARANCE
MAXI-TUSS DM
chlorpheniramine maleate and dextromethorphan hydrobromide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 58605-304 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 4 mg in 5 mL Dextromethorphan Hydrobromide (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) Dextromethorphan Hydrobromide 18 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Methylparaben (UNII: A2I8C7HI9T) Potassium Citrate (UNII: EE90ONI6FF) Potassium Sorbate (UNII: 1VPU26JZZ4) Propylene Glycol (UNII: 6DC9Q167V3) Propylparaben (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) Sorbitol (UNII: 506T60A25R) Sucralose (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor TUTTI FRUTTI Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58605-304-16 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/01/2018 2 NDC: 58605-304-10 10 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 04/01/2018 Labeler - MCR American Pharmaceuticals, Inc. (783383011) Establishment Name Address ID/FEI Business Operations MCR American Pharmaceuticals, Inc. 783383011 MANUFACTURE(58605-304)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.