0.9 percent saline solution
0.9 percent saline solution by
Drug Labeling and Warnings
0.9 percent saline solution by is a Otc medication manufactured, distributed, or labeled by Guangzhou Jinsheng Biotechnology Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
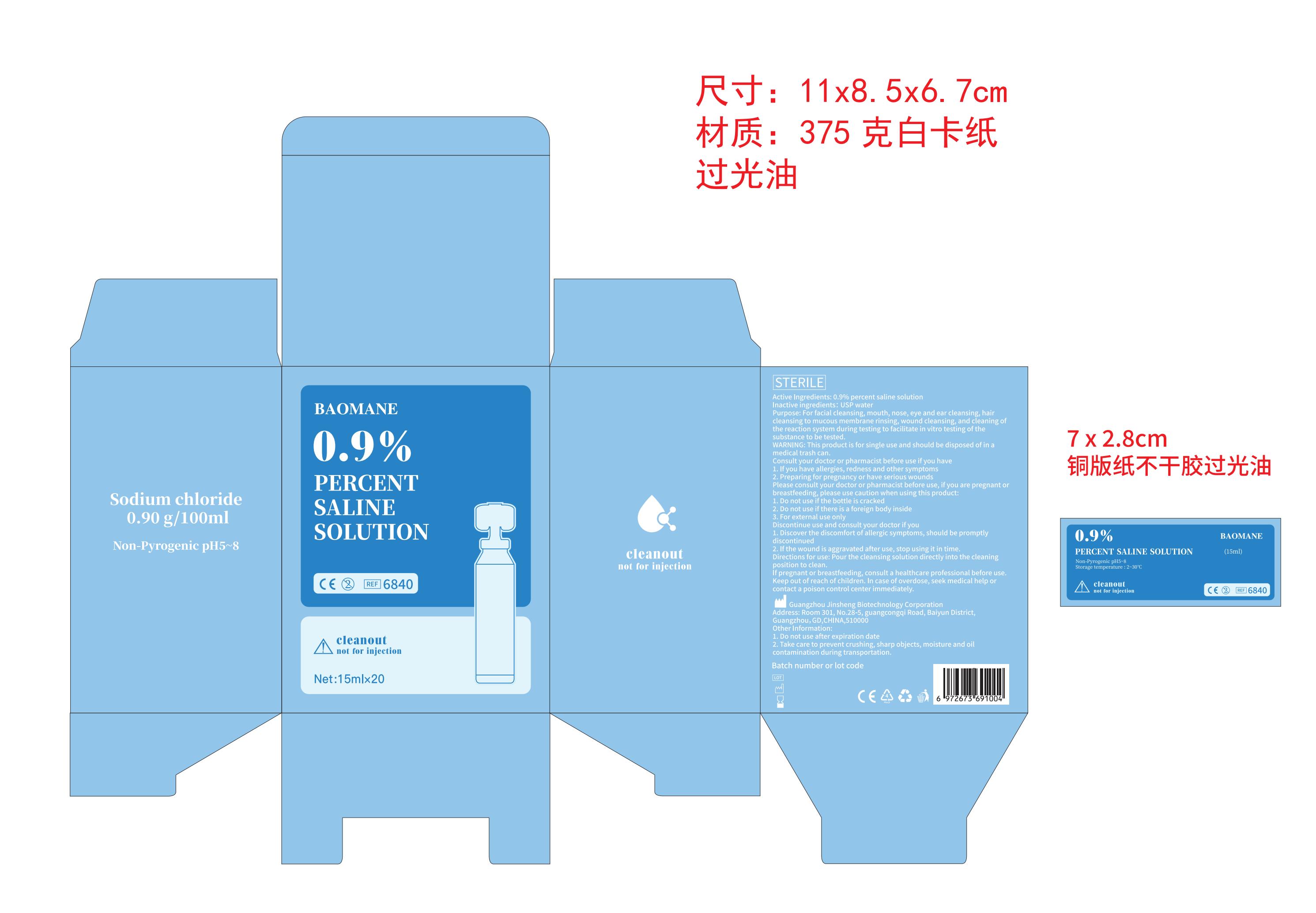
0.9 PERCENT SALINE SOLUTION- sodium chloride liquid
Guangzhou Jinsheng Biotechnology Corporation
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
0.9 percent saline solution
Warings:
NOT FOR INJECTION
This product is for single use and should be disposed of in amedical trash can.
Keep out of reach of children.
In case of overdose, seek medical help or contact a poison control center immediately.
Purpose: For facial cleansing, mouth, nose, eye and ear cleansing, hair cleansing to mucous membrane rinsing, wound cleansing, and cleaning of the reaction system during testing to facilitate in vitro testing of the substance to be tested.
For facial cleansing, mouth, nose, eye and ear cleansing, hair cleansing to mucous membrane rinsing, wound cleansing, and cleaning of the reaction system during testing to facilitate in vitro testing of the substance to be tested.
Pour the cleansing solution directly onto the affected area to clean.
Consult your doctor or pharmacist before use if you have
1. If you have allergies, redness and other symptoms
2. Preparing for pregnancy or have serious wounds
Please consult your doctor or pharmacist before use, if you are pregnant or breastfeeding, please use caution when using this product:
1. Do not use if the bottle is cracked
2. Do not use if there is a foreign body inside
3. For external use only
Discontinue use and consult your doctor if you
1. Discover the discomfort of allergic symptoms, should be promptly discontinued
2. If the wound is aggravated after use, stop using it in time.
| 0.9 PERCENT SALINE SOLUTION
sodium chloride liquid |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Guangzhou Jinsheng Biotechnology Corporation (402822781) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Guangzhou Jinsheng Biotechnology Corporation | 402822781 | manufacture(85797-101) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 See Label
See Label