HYPERTET S/D (tetanus immune globulin- human injection
HyperTET by
Drug Labeling and Warnings
HyperTET by is a Other medication manufactured, distributed, or labeled by GRIFOLS USA, LLC, Grifols Therapeutics LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Tetanus Immune Globulin (Human) — HyperTET® S/D treated with solvent/detergent is a colorless to pale yellow or pink sterile solution of tetanus hyperimmune immune globulin for intramuscular administration; it is preservative-free, in a latex-free delivery system. HyperTET S/D is prepared by cold ethanol fractionation from the plasma of donors immunized with tetanus toxoid. The immune globulin is isolated from solubilized Cohn Fraction II. The Fraction II solution is adjusted to a final concentration of 0.3% tri-n-butyl phosphate (TNBP) and 0.2% sodium cholate. After the addition of solvent (TNBP) and detergent (sodium cholate), the solution is heated to 30°C and maintained at that temperature for not less than 6 hours. After the viral inactivation step, the reactants are removed by precipitation, filtration and finally ultrafiltration and diafiltration. HyperTET S/D is formulated as a 15–18% protein solution at a pH of 6.4–7.2 in 0.21–0.32 M glycine. HyperTET S/D is then incubated in the final container for 21–28 days at 20–27°C. The product is standardized against the U.S. Standard Antitoxin and the U.S. Control Tetanus Toxin and contains not less than 250 tetanus antitoxin units per container.
The removal and inactivation of spiked model enveloped and non-enveloped viruses during the manufacturing process for HyperTET S/D has been validated in laboratory studies. Human Immunodeficiency Virus, Type 1 (HIV-1), was chosen as the relevant virus for blood products; Bovine Viral Diarrhea Virus (BVDV) was chosen to model Hepatitis C virus; Pseudorabies virus (PRV) was chosen to model Human Herpes viruses and other large enveloped DNA viruses; and Reo virus type 3 (Reo) was chosen to model non-enveloped viruses and for its resistance to physical and chemical inactivation. Significant removal of model enveloped and non-enveloped viruses is achieved at two steps in the Cohn fractionation process leading to the collection of Cohn Fraction II: the precipitation and removal of Fraction III in the processing of Fraction II + IIIW suspension to Effluent III and the filtration step in the processing of Effluent III to Filtrate III. Significant inactivation of enveloped viruses is achieved at the time of treatment of solubilized Cohn Fraction II with TNBP/sodium cholate.
Additionally, the manufacturing process was investigated for its capacity to decrease the infectivity of an experimental agent of transmissible spongiform encephalopathy (TSE), considered as a model for the vCJD and CJD agents. [18-21]
Studies of the HyperTET S/D manufacturing process demonstrate that TSE clearance is achieved during the Pooled Plasma to Effluent III Fractionation Process (6.7 log10). These studies provide reasonable assurance that low levels of CJD/vCJD agent infectivity, if present in the starting material, would be removed.
-
CLINICAL PHARMACOLOGY
The occurrence of tetanus in the United States has decreased dramatically from 560 reported cases in 1947, when national reporting began, to a record low of 48 reported cases in 1987. [1] The decline has resulted from widespread use of tetanus toxoid and improved wound management, including use of tetanus prophylaxis in emergency rooms. [2]
HyperTET S/D supplies passive immunity to those individuals who have low or no immunity to the toxin produced by the tetanus organism, Clostridium tetani. The antibodies act to neutralize the free form of the powerful exotoxin produced by this bacterium. Historically, such passive protection was provided by antitoxin derived from equine or bovine serum; however, the foreign protein in these heterologous products often produced severe allergic manifestations, even in individuals who demonstrated negative skin and/or conjunctival tests prior to administration. Estimates of the frequency of these foreign protein reactions following antitoxin of equine origin varied from 5%–30%.[3-6] If passive immunization is needed, human tetanus immune globulin (TIG) is the product of choice. It provides protection longer than antitoxin of animal origin and causes few adverse reactions. [2]
Several studies suggest the value of human tetanus antitoxin in the treatment of active tetanus. [7,8] In 1961 and 1962, Nation et al, [7] using Hyper-Tet treated 20 patients with tetanus using single doses of 3,000 to 6,000 antitoxin units in combination with other accepted clinical and nursing procedures. Six patients, all over 45 years of age, died of causes other than tetanus. The authors felt that the mortality rate (30%) compared favorably with their previous experience using equine antitoxin in larger doses and that the results were much better than the 60% national death rate for tetanus reported from 1951 to 1954. [9] Blake et al, [10] however, found in a data analysis of 545 cases of tetanus reported to the Centers for Disease Control from 1965 to 1971 that survival was no better with 8,000 units of TIG than with 500 units; however, an optimal dose could not be determined.
Serologic tests indicate that naturally acquired immunity to tetanus toxin does not occur in the United States. Thus, universal primary vaccination, with subsequent maintenance of adequate antitoxin levels by means of appropriately timed boosters, is necessary to protect persons among all age groups. Tetanus toxoid is a highly effective antigen; a completed primary series generally induces protective levels of serum antitoxin that persist for ≥10 years. [2]
Passive immunization with HyperTET S/D may be undertaken concomitantly with active immunization using tetanus toxoid in those persons who must receive an immediate injection of tetanus antitoxin and in whom it is desirable to begin the process of active immunization. Based on the work of Rubbo, [11] McComb and Dwyer, [12] and Levine et al, [13] the physician may thus supply immediate passive protection against tetanus, and at the same time begin formation of active immunization in the injured individual which upon completion of a full toxoid series will preclude future need for antitoxin.
Peak blood levels of IgG are obtained approximately 2 days after intramuscular injection. The half-life of IgG in the circulation of individuals with normal IgG levels is approximately 23 days. [14]
In a clinical study in eight healthy human adults receiving another hyperimmune immune globulin product treated with solvent/detergent, Rabies Immune Globulin (Human), HyperRAB® S/D, prepared by the same manufacturing process, detectable passive antibody titers were observed in the serum of all subjects by 24 hours post injection and persisted through the 21 day study period. These results suggest that passive immunization with immune globulin products is not affected by the solvent/detergent treatment.
-
INDICATIONS AND USAGE
HyperTET S/D is indicated for prophylaxis against tetanus following injury in patients whose immunization is incomplete or uncertain (see below). It is also indicated, although evidence of effectiveness is limited, in the regimen of treatment of active cases of tetanus. [7,8,15]
A thorough attempt must be made to determine whether a patient has completed primary vaccination. Patients with unknown or uncertain previous vaccination histories should be considered to have had no previous tetanus toxoid doses. Persons who had military service since 1941 can be considered to have received at least one dose, and although most of them may have completed a primary series of tetanus toxoid, this cannot be assumed for each individual. Patients who have not completed a primary series may require tetanus toxoid and passive immunization at the time of wound cleaning and debridement. [2]
The following table is a summary guide to tetanus prophylaxis in wound management:
Guide to Tetanus Prophylaxis in Wound Management [2] - * Such as, but not limited to, wounds contaminated with dirt, feces, soil, and saliva; puncture wounds; avulsions; and wounds resulting from missiles, crushing, burns and frostbite.
- † Adult type tetanus and diphtheria toxoids. If the patient is less than 7 years old, DT or DTP is preferred to tetanus toxoid alone. For persons ≥7 years of age, Td is preferred to tetanus toxoid alone. (see Dosage and Administration )
- ‡ Tetanus Immune Globulin (Human).
- § If only three doses of fluid tetanus toxoid have been received, a fourth dose of toxoid, preferably an adsorbed toxoid, should be given.
- ¶ Yes if more than 10 years since the last dose.
- # Yes if more than 5 years since the last dose. (More frequent boosters are not needed and can accentuate side effects).
History of Tetanus Immunization
(Doses)Clean, Minor Wounds All Other Wounds* Td† TIG‡ Td TIG Uncertain or less than 3 Yes No Yes Yes 3 or more§ No¶ No No# No - CONTRAINDICATIONS
-
WARNINGS
HyperTET S/D is made from human plasma. Products made from human plasma may contain infectious agents, such as viruses, and, theoretically, the Creutzfeldt-Jakob Disease (CJD) agent that can cause disease. The risk that such products will transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and/or removing certain viruses. Despite these measures, such products can still potentially transmit disease. There is also the possibility that unknown infectious agents may be present in such products. Individuals who receive infusions of blood or plasma products may develop signs and/or symptoms of some viral infections, particularly hepatitis C. ALL infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Grifols Therapeutics LLC [1-800-520-2807].
The physician should discuss the risks and benefits of this product with the patient, before prescribing or administering it to the patient.
HyperTET S/D should be given with caution to patients with a history of prior systemic allergic reactions following the administration of human immunoglobulin preparations.
In patients who have severe thrombocytopenia or any coagulation disorder that would contraindicate intramuscular injections, HyperTET S/D should be given only if the expected benefits outweigh the risks.
-
PRECAUTIONS
General
HyperTET S/D should not be given intravenously. Intravenous injection of immunoglobulin intended for intramuscular use can, on occasion, cause a precipitous fall in blood pressure, and a picture not unlike anaphylaxis. Injections should only be made intramuscularly and care should be taken to draw back on the plunger of the syringe before injection in order to be certain that the needle is not in a blood vessel. Intramuscular injections are preferably administered in the deltoid muscle of the upper arm or lateral thigh muscle. The gluteal region should not be used as an injection site because of the risk of injury to the sciatic nerve. [16]
Chemoprophylaxis against tetanus is neither practical nor useful in managing wounds. Wound cleaning, debridement when indicated, and proper immunization are important. The need for tetanus toxoid (active immunization), with or without TIG (passive immunization), depends on both the condition of the wound and the patient’s vaccination history. Rarely has tetanus occurred among persons with documentation of having received a primary series of toxoid injections. [2] See table under INDICATIONS AND USAGE.
Skin tests should not be done. The intradermal injection of concentrated IgG solutions often causes a localized area of inflammation which can be misinterpreted as a positive allergic reaction. In actuality, this does not represent an allergy; rather, it is localized tissue irritation. Misinterpretation of the results of such tests can lead the physician to withhold needed human antitoxin from a patient who is not actually allergic to this material. True allergic responses to human IgG given in the prescribed intramuscular manner are rare.
Although systemic reactions to human immunoglobulin preparations are rare, epinephrine should be available for treatment of acute anaphylactic reactions.
Drug Interactions
Antibodies in immunoglobulin preparations may interfere with the response to live viral vaccines such as measles, mumps, polio, and rubella. Therefore, use of such vaccines should be deferred until approximately 3 months after Tetanus Immune Globulin (Human) — HyperTET® S/D administration.
No interactions with other products are known.
-
ADVERSE REACTIONS
Slight soreness at the site of injection and slight temperature elevation may be noted at times. Sensitization to repeated injections of human immunoglobulin is extremely rare.
In the course of routine injections of large numbers of persons with immunoglobulin there have been a few isolated occurrences of angioneurotic edema, nephrotic syndrome, and anaphylactic shock after injection.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Routine prophylactic dosage schedule:
-
Adults and children 7 years and older: HyperTET S/D, 250 units should be given by deep intramuscular injection (see PRECAUTIONS). At the same time, but in a different extremity and with a separate syringe, Tetanus and Diphtheria Toxoids Adsorbed (For Adult Use) (Td) should be administered according to the manufacturer's package insert. Adults with uncertain histories of a complete primary vaccination series should receive a primary series using the combined Td toxoid. To ensure continued protection, booster doses of Td should be given every 10 years. [2]
-
Children less than 7 years old: In small children the routine prophylactic dose of HyperTET S/D may be calculated by the body weight (4.0 units/kg). However, it may be advisable to administer the entire contents of the syringe of HyperTET S/D (250 units) regardless of the child's size, since theoretically the same amount of toxin will be produced in the child's body by the infecting tetanus organism as it will in an adult's body. At the same time but in a different extremity and with a different syringe, Diphtheria and Tetanus Toxoids and Pertussis Vaccine Adsorbed (DTP) or Diphtheria and Tetanus Toxoids Adsorbed (For Pediatric Use) (DT), if pertussis vaccine is contraindicated, should be administered per the manufacturer's package insert.
-
Note: The single injection of tetanus toxoid only initiates the series for producing active immunity in the recipient. The physician must impress upon the patient the need for further toxoid injections in 1 month and 1 year. Without such, the active immunization series is incomplete. If a contraindication to using tetanus toxoid-containing preparations exists for a person who has not completed a primary series of tetanus toxoid immunization and that person has a wound that is neither clean nor minor, only passive immunization should be given using tetanus immune globulin. [2] See table under INDICATIONS AND USAGE.
-
Available evidence indicates that complete primary vaccination with tetanus toxoid provides long lasting protection ≥10 years for most recipients. Consequently, after complete primary tetanus vaccination, boosters-even for wound management-need be given only every 10 years when wounds are minor and uncontaminated. For other wounds, a booster is appropriate if the patient has not received tetanus toxoid within the preceding 5 years. Persons who have received at least two doses of tetanus toxoid rapidly develop antibodies. [2] The prophylactic dosage schedule for these patients and for those with incomplete or uncertain immunity is shown on the table in INDICATIONS AND USAGE.
-
Since tetanus is actually a local infection, proper initial wound care is of paramount importance. The use of antitoxin is adjunctive to this procedure. However, in approximately 10% of recent tetanus cases, no wound or other breach in skin or mucous membrane could be implicated. [17]
Treatment of active cases of tetanus:
-
Standard therapy for the treatment of active tetanus including the use of HyperTET S/D must be implemented immediately. The dosage should be adjusted according to the severity of the infection. [7,8]
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. They should not be used if particulate matter and/or discoloration are present.
HyperTET S/D is supplied with a syringe and an attached UltraSafe® Needle Guard for your protection and convenience. Please follow instructions below for proper use of syringe and UltraSafe® Needle Guard.
Directions for Syringe Usage
- Remove the prefilled syringe from the package. Lift syringe by barrel, not by plunger.
- Twist the plunger rod clockwise until the threads are seated.
- With the rubber needle shield secured on the syringe tip, push the plunger rod forward a few millimeters to break any friction seal between the rubber stopper and the glass syringe barrel.
- Remove the needle shield and expel air bubbles. [Do not remove the rubber needle shield to prepare the product for administration until immediately prior to the anticipated injection time.]
- Proceed with hypodermic needle puncture.
- Aspirate prior to injection to confirm that the needle is not in a vein or artery.
- Inject the medication.
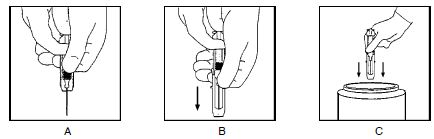
- Keeping your hands behind the needle, grasp the guard with free hand and slide forward toward needle until it is completely covered and guard clicks into place. If audible click is not heard, guard may not be completely activated. (See Diagrams A and B)
- Place entire prefilled glass syringe with guard activated into an approved sharps container for proper disposal. (See Diagram C )
A number of factors could reduce the efficacy of this product or even result in an ill effect following its use. These include improper storage and handling of the product after it leaves our hands, diagnosis, dosage, method of administration, and biological differences in individual patients. Because of these factors it is important that this product be stored properly and that the directions be followed carefully during use.
-
- HOW SUPPLIED
- STORAGE
- CAUTION
-
REFERENCES
- Tetanus — United States, 1987 and 1988, MMWR 39(3): 37-41, 1990.
- Diphtheria, Tetanus, and Pertussis: Recommendations for Vaccine Use and Other Preventive Measures. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR 40 (RR-10): 1-28, 1991.
- Moynihan NH: Tetanus prophylaxis and serum sensitivity tests. Br Med J 1:260-4, 1956.
- Scheibel I: The uses and results of active tetanus immunization. Bull WHO 13:381-94, 1955.
- Edsall G: Specific prophylaxis of tetanus. JAMA 171(4):417-27, 1959.
- Bardenwerper HW: Serum neuritis from tetanus antitoxin. JAMA 179(10):763-6, 1962.
- Nation NS, Pierce NF, Adler SJ, et al: Tetanus: the use of human hyperimmune globulin in treatment. Calif Med 98(6):305-6, 1963.
- Ellis M: Human antitetanus serum in the treatment of tetanus. Br Med J 1(5338):1123-6, 1963.
- Axnick NW, Alexander ER: Tetanus in the United States: A review of the problem. Am J Public Health 47(12):1493-1501, 1957.
- Blake PA, Feldman RA, Buchanan TM, et al: Serologic therapy of tetanus in the United States, 1965-1971. JAMA 235(1):42-4, 1976.
- Rubbo SD: New approaches to tetanus prophylaxis. Lancet 2(7461):449-53, 1966.
- McComb JA, Dwyer RC: Passive-active immunization with tetanus immune globulin (human). N Engl J Med 268(16):857-62, 1963.
- Levine L, McComb JA, Dwyer RC, et al: Active-passive tetanus immunization; choice of toxoid, dose of tetanus immune globulin and timing of injections. N Engl J Med 274(4):186-90, 1966.
- Waldmann TA, Strober W, Blaese RM: Variations in the metabolism of immunoglobulins measured by turnover rates. In Merler E (ed.): Immunoglobulins: biologic aspects and clinical uses. Washington, DC, Nat Acad Sci, 1970, p. 33-51.
- McCracken GH Jr., Dowell DL, Marshall FN: Double-blind trial of equine antitoxin and human immune globulin in tetanus neonatorum. Lancet 1(7710):1146-9, 1971.
- Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP): General recommendations on immunization. MMWR 2002: 51(RR02), 1-36.
- Tetanus-Rates by year, United States, 1955-1984. Annual Summary 1984. MMWR 33 (54):61, 1986.
- Stenland CJ, Lee DC, Brown P, et al. Partitioning of human and sheep forms of the pathogenic prion protein during the purification of therapeutic proteins from human plasma. Transfusion 2002. 42(11):1497-500.
- Lee DC, Stenland CJ, Miller JL, et al. A direct relationship between the partitioning of the pathogenic prion protein and transmissible spongiform encephalopathy infectivity during the purification of plasma proteins. Transfusion 2001. 41(4):449-55.
- Lee DC, Stenland CJ, Hartwell RC, et al. Monitoring plasma processing steps with a sensitive Western blot assay for the detection of the prion protein. J Virol Methods 2000. 84(1):77-89.
- Cai K, Miller JL, Stenland CJ, et al. Solvent-dependent precipitation of prion protein. Biochim Biophys Acta 2002. 1597(1):28-35.
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
U.S. License No. 18713051851
(Rev. 6/2018) -
PACKAGE LABEL
Tetanus Immune Globulin (Human)
HyperTET® S/D
Solvent/Detergent Treated
Preservative-free, latex-free delivery system
250 Units
Contents: One single dose disposable syringe with attached needle.
Tetanus Immune Globulin (Human) is a sterile solution of immunoglobulin containing 15%–18% protein stabilized with 0.21–0.32M glycine. The pH is adjusted with sodium carbonate.
The potency of each syringe is not less than 250 antitoxin units based on the U.S. Standard Antitoxin and the U.S. Control Tetanus Toxin.
FOR INTRAMUSCULAR INJECTION ONLY. DO NOT GIVE INTRAVENOUSLY.
Store at 2–8°C (36–46°F).
Do not freeze.
NDC 13533-634-02
GRIFOLS
The patient and physician should discuss the risks and benefits of this product.
For complete dosage and administration information, read enclosed package insert.
For directions for syringe usage, see enclosed package insert.
Do not use if the syringe is prematurely engaged.
Not returnable for credit or exchange.
Rx only
CAUTION: U.S. federal law prohibits dispensing without prescription.
Grifols Therapeutics LLC
Research Triangle Park,
NC 27709 USA
U.S. License No. 1871
Carton: 3045905
GTIN 00313533634025
LOT
XXXXXXXXXX
EXP
DDMMMYYYY
SN
XXXXXXXXXXXXXX

Tetanus Immune Globulin (Human).
HyperTET® S/D
Solvent/Detergent Treated
250 Units
Grifols Therapeutics LLC
Research Triangle Park,
NC 27709 USA
U.S. License No. 1871
The patient and physician should discuss the risks and benefits of this product.
3051845
Lot
Exp.

-
INGREDIENTS AND APPEARANCE
HYPERTET S/D
tetanus immune globulin (human) injectionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 13533-634 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Human Clostridium Tetani Toxoid Immune Globulin (UNII: V4SWI4RF4J) (Human Clostridium Tetani Toxoid Immune Globulin - UNII:V4SWI4RF4J) Human Clostridium Tetani Toxoid Immune Globulin 250 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength Glycine (UNII: TE7660XO1C) Water (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (Clear liquid, colorless to pale yellow or pink) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13533-634-02 1 in 1 BOX 1 NDC: 13533-634-20 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101142 08/14/1996 Labeler - GRIFOLS USA, LLC (048987452) Establishment Name Address ID/FEI Business Operations Grifols Therapeutics LLC 611019113 manufacture(13533-634)
Trademark Results [HyperTET]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HYPERTET 78622911 3202846 Live/Registered |
GRIFOLS THERAPEUTICS LLC 2005-05-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.