LIDOCAINE 5 PERCENT- lidocaine cream
Lidocaine 5 Percent by
Drug Labeling and Warnings
Lidocaine 5 Percent by is a Otc medication manufactured, distributed, or labeled by Alexso, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SPL UNCLASSIFIED SECTION
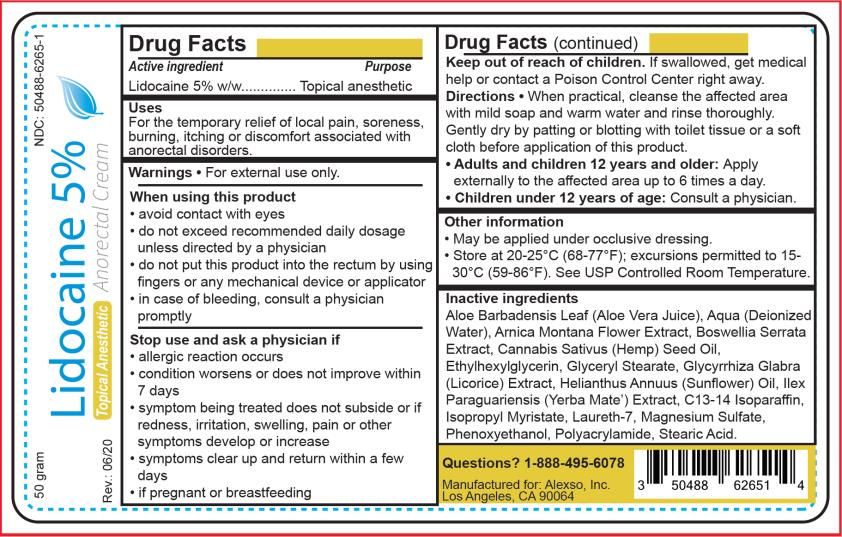
LIDOCAINE - Lidocaine 5% anorectal cream
Alexso, Inc.Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Lidocaine 5% Anorectal Cream
Drug Facts
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only.
When using this product
- avoid contact with eyes
- do not exceed recommended daily dosage unless directed by a physician
- do not put this product into the rectum by using fingers or any mechanical device or applicator
- in case of bleeding, consult a physician promptly
Stop use and ask a physician if
- allergic reaction occurs
- condition worsens or does not improve within 7 days
- symptom being treated does not subside or if redness, irritation, swelling, pain or other symptoms develop or increase
- symptoms clear up and return within a few days
- if pregnant or breastfeeding
- avoid contact with eyes
-
Directions
When practical, cleanse the affected area with mild soap and warm water and rinse thoroughly. Gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product.
Adults and children 12 years and older: Apply externally to the affected area up to 6 times a day.
Children under 12 years of age: Consult a physician. - Other information
-
Inactive ingredients
Aloe Barbadensis Leaf (Aloe Vera Juice), Aqua (Deionized Water), Arnica Montana Flower Extract, Boswellia Serrata Extract, Cannabis Sativus (Hemp) Seed Oil, Ethylhexylglycerin, Glyceryl Stearate, Glycyrrhiza Glabra (Licorice) Extract, Helianthus Annuus (Sunflower) Oil, Ilex Paraguariensis (Yerba Mate) Extract, C13-14 Isoparaffin, Isopropyl Myristate, Laureth-7, Magnesium Sulfate, Phenoxyethanol, Polyacrylamide, Stearic Acid.
-
PRINCIPAL DISPLAY PANEL
Lidocaine 5% Cream
NDC: 50488-6265-1
Lidocaine 5%
Anorectal Cream
Topical Anesthetic
50 grams
Manufactured for:
Alexso, Inc.
Los Angeles, CA 90064
-
INGREDIENTS AND APPEARANCE
LIDOCAINE 5 PERCENT
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 50488-6265 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 5 g in 100 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) SUNFLOWER OIL (UNII: 3W1JG795YI) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) LAURETH-7 (UNII: Z95S6G8201) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) POLYACRYLAMIDE (10000 MW) (UNII: E2KR9C9V2I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50488-6265-1 1 in 1 CARTON 07/01/2020 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part346 07/01/2020 Labeler - Alexso, Inc (963338061)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.