FLUIDBASE AHA 10 by LABORATORIO GENOVE S.A. FLUID BASE LOTION 10 AHA
FLUIDBASE AHA 10 by
Drug Labeling and Warnings
FLUIDBASE AHA 10 by is a Otc medication manufactured, distributed, or labeled by LABORATORIO GENOVE S.A.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
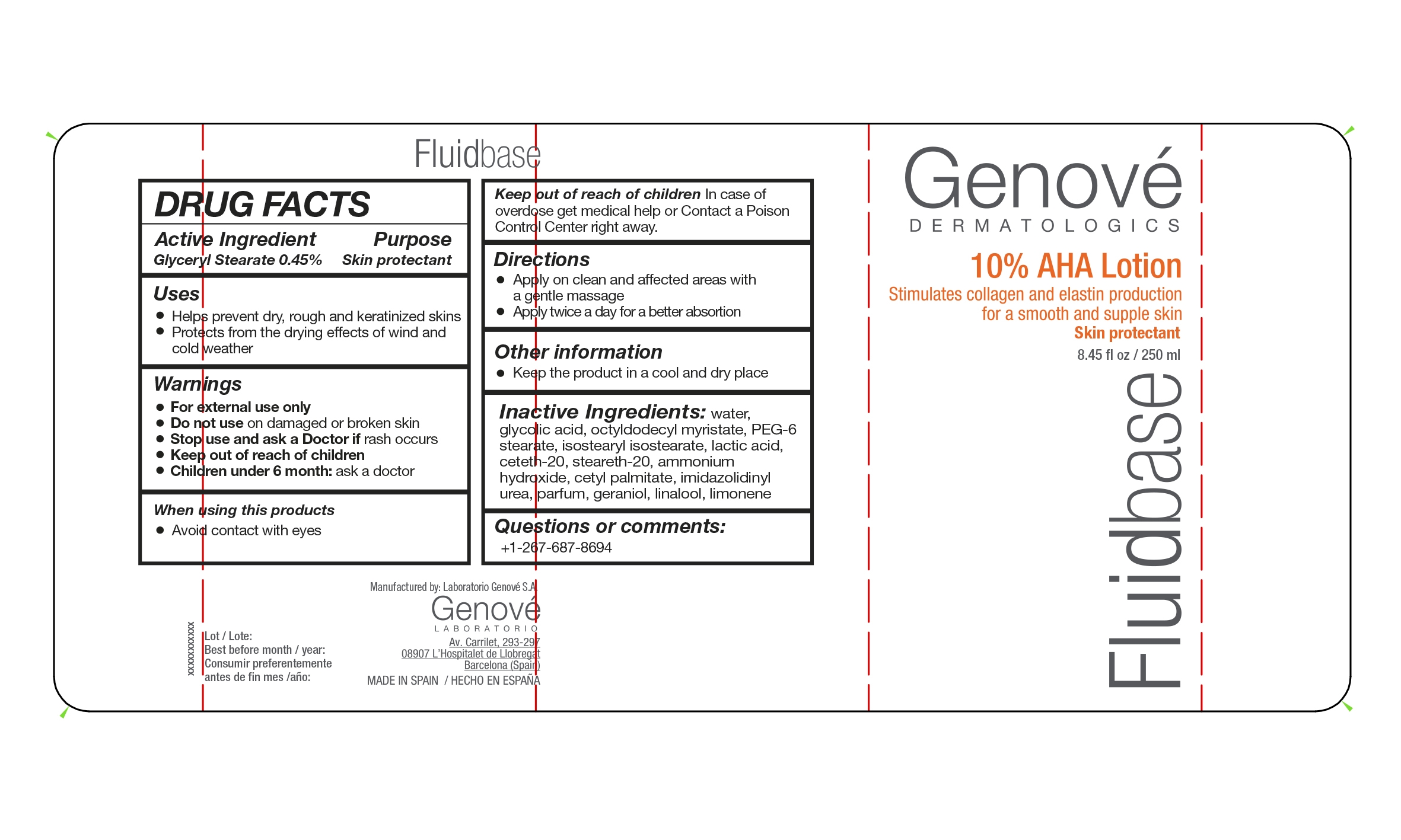
FLUIDBASE AHA 10- glyceryl stearate lotion
LABORATORIO GENOVE S.A.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
FLUID BASE LOTION 10 AHA
INACTIVE INGREDIENTS
water,glycolic acid, octyldodecyl myristate, PEG-6 stearate, isostearyl isostearate, lactic acid, ceteth-20, steareth-20, ammonia, cetyl palmitate, imidurea, perflunafene, geraniol, linalool, limonene

USES
- helps prevent dry, rough and keratinized skin
- protects from the drying effects of wind and cold weather
WARNINGS
- For external use only
- Do not use on damaged or broken skin
- Stop use and ask a Doctor is rash occurs
- Keep out of reach of children
- Children under 6 months: ask a doctor
WARNINGS
Keep out of reach of children. In case of overdose
get medical help or contact a Poison Control Center
right away.
DIRECTIONS
- Apply on a clean and affected areas with a gentle massage
- Apply twice a day for a better absortion
DIRECTIONS
- Apply on a clean and affected areas with a gentle massage
- Apply twice a day for a better absortion
| FLUIDBASE AHA 10
glyceryl stearate lotion |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - LABORATORIO GENOVE S.A. (464955435) |
| Registrant - LABORATORIO GENOVE S.A. (464955435) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LABORATORIO GENOVE S.A. | 464955435 | manufacture(70963-002) | |
Revised: 2/2020
Document Id: 9e75d395-218b-3027-e053-2995a90a438f
Set id: 3956632c-c6af-2a0f-e054-00144ff8d46c
Version: 2
Effective Time: 20200213
LABORATORIO GENOVE S.A.