These highlights do not include all the information needed to use OLUX Foam safely and effectively. See full prescribing information for OLUX Foam. OLUX (clobetasol propionate) Foam, 0.05% for topical useInitial U.S. Approval: 1985
OLUX by
Drug Labeling and Warnings
OLUX by is a Prescription medication manufactured, distributed, or labeled by Prestium Pharma, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
OLUX- clobetasol propionate aerosol, foam
Prestium Pharma, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use OLUX Foam safely and effectively. See full prescribing information for OLUX Foam.
OLUX (clobetasol propionate) Foam, 0.05% for topical use Initial U.S. Approval: 1985 INDICATIONS AND USAGEOLUX Foam is a corticosteroid indicated for treatment of moderate to severe plaque psoriasis of the scalp and mild to moderate plaque psoriasis of non-scalp regions of the body excluding the face and intertriginous areas in patients 12 years and older. (1) DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions (≥ 4%) are application site burning and other application site reactions. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Prestium Pharma, Inc. at 1-866-897-5002 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 10/2014 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
OLUX Foam is a corticosteroid indicated for treatment of moderate to severe plaque psoriasis of the scalp and mild to moderate plaque psoriasis of non-scalp regions of the body excluding the face and intertriginous areas in patients 12 years and older.
2 DOSAGE AND ADMINISTRATION
Apply a thin layer of OLUX Foam to the affected skin areas twice daily.
OLUX Foam is a super-high-potency topical corticosteroid; therefore treatment should be limited to 2 consecutive weeks and patients should not use greater than 50 grams per week or more than 21 capfuls per week because of the potential for the drug to suppress the hypothalamic-pituitary-adrenal (HPA) axis.
Therapy should be discontinued when control is achieved.
OLUX Foam should not be used with occlusive dressings unless directed by a physician.
OLUX Foam is for topical use only. It is not for oral, ophthalmic, or intravaginal use.
Avoid use on the face, groin, or axillae, or if skin atrophy is present at the treatment site.
3 DOSAGE FORMS AND STRENGTHS
Foam, 0.05%. Each gram of OLUX Foam contains 0.5 mg of clobetasol propionate in a white aerosol foam.
5 WARNINGS AND PRECAUTIONS
5.1 Effects on Endocrine System
OLUX Foam can cause reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency. This may occur during treatment or after withdrawal of treatment. Factors that predispose a patient to HPA axis suppression include the use of high-potency steroids, large treatment surface areas, prolonged use, use of occlusive dressings, altered skin barrier, liver failure, and young age. Evaluation for HPA axis suppression may be done by using the adrenocorticotropic hormone (ACTH) stimulation test.
In a trial evaluating the effects of OLUX Foam on the HPA axis, 13 subjects applied OLUX Foam to at least 20% of involved body surface area for 14 days. HPA axis suppression was identified in 5 out of 13 subjects (38%) [see Clinical Pharmacology (12.2)].
If HPA axis suppression is documented, gradually withdraw the drug, reduce the frequency of application, or substitute with a less potent corticosteroid.
Cushing’s syndrome and hyperglycemia may also occur due to the systemic effects of the topical corticosteroid. These complications are rare and generally occur after prolonged exposure to excessively large doses, especially of high-potency topical corticosteroids.
Pediatric patients may be more susceptible to systemic toxicity due to their larger skin surface to body mass ratios [see Use in Specific Populations (8.4)].
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In a controlled clinical trial involving 188 subjects with psoriasis of the scalp, there were no localized scalp adverse reactions reported in the subjects treated with OLUX Foam. In 2 controlled clinical trials with OLUX Foam in 360 subjects with psoriasis of non-scalp regions, localized adverse events that occurred in the subjects treated with OLUX Foam included application site burning (10%), application site dryness (<1%), and other application site reactions (4%).
In larger controlled trials with other clobetasol propionate formulations, the most frequently reported local adverse reactions have included burning, stinging, irritation, pruritus, erythema, folliculitis, cracking and fissuring of the skin, numbness of the fingers, skin atrophy, and telangiectasia (all less than 2%).
6.2 Postmarketing Experience
Because adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Postmarketing reports for local adverse reactions to topical corticosteroids may also include: striae, itching, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, hypertrichosis, and miliaria.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects
Pregnancy Category C.
There are no adequate and well-controlled studies in pregnant women. OLUX Foam should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically. Some corticosteroids have been shown to be teratogenic after dermal application to laboratory animals.
Clobetasol propionate has not been tested for teratogenicity when applied topically; however, it is absorbed percutaneously, and when administered subcutaneously, it was a significant teratogen in both the rabbit and the mouse. Clobetasol propionate has greater teratogenic potential than steroids that are less potent.
Teratogenicity studies in mice using the subcutaneous route resulted in fetotoxicity at the highest dose tested (1 mg/kg) and teratogenicity at all dose levels tested down to 0.03 mg/kg. These doses are approximately 1.4 and 0.04 times, respectively, the human topical dose of OLUX Foam based on body surface area comparisons. Abnormalities seen included cleft palate and skeletal abnormalities.
In rabbits, clobetasol propionate was teratogenic at doses of 0.003 and 0.01 mg/kg. These doses are approximately 0.02 and 0.05 times, respectively, the human topical dose of OLUX Foam based on body surface area comparisons. Abnormalities seen included cleft palate, cranioschisis, and other skeletal abnormalities.
8.3 Nursing Mothers
Systemically administered corticosteroids appear in human milk and can suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids can result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when OLUX Foam is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness of OLUX Foam in patients younger than 12 years of age have not been established; therefore, use in children younger than 12 years is not recommended.
Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of systemic toxicity when they are treated with topical drugs. They are, therefore, also at greater risk of adrenal insufficiency upon the use of topical corticosteroids.
Rare systemic toxicities such as Cushing’s syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in pediatric patients especially those with prolonged exposure to large doses of high potency topical corticosteroids.
Local adverse reactions including striae have also been reported with use of topical corticosteroids in pediatric patients.
Avoid use of OLUX Foam in the treatment of diaper dermatitis.
8.5 Geriatric Use
Clinical studies of OLUX Foam did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range.
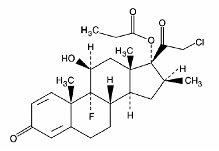
11 DESCRIPTION
OLUX (clobetasol propionate) Foam, 0.05%, is a white thermolabile hydroethanolic aerosol foam containing the active ingredient, clobetasol propionate, USP, a synthetic corticosteroid, for topical use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree of mineralocorticoid activity.
Clobetasol propionate is 21-chloro-9-fluoro-11ß,17-dihydroxy-16ß-methylpregna-1,4-diene-3,20-dione 17-propionate, with the empirical formula C25H32CIFO5, a molecular weight of 466.97.
The following is the chemical structure:
Clobetasol propionate is a white to cream-colored crystalline powder, practically insoluble in water.
Each gram of OLUX Foam contains 0.5 mg clobetasol propionate, USP. The foam also contains cetyl alcohol, citric acid, ethanol (60%), polysorbate 60, potassium citrate, propylene glycol, purified water, and stearyl alcohol pressurized with a hydrocarbon (propane/butane) propellant.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Corticosteroids play a role in cellular signaling, immune function, inflammation, and protein regulation; however, the precise mechanism of action in corticosteroid-responsive dermatoses is unknown.
The contribution to efficacy by individual components of the vehicle has not been established.
12.2 Pharmacodynamics
In a controlled pharmacokinetic trial, 5 of 13 subjects experienced reversible suppression of the adrenals at any time during the 14 days of therapy with OLUX Foam applied to at least 20% of involved body surface area. Of the 13 subjects studied, 1 of 9 with psoriasis was suppressed after 14 days and all 4 of the subjects with atopic dermatitis had abnormal cortisol levels indicative of adrenal suppression at some time after starting therapy with OLUX Foam (See Table 1 below).
|
|
|
|
Dermatosis |
OLUX Foam |
|
Psoriasis |
1 of 9 |
|
Atopic Dermatitis* |
4 of 4 |
12.3 Pharmacokinetics
Topical corticosteroids can be absorbed from intact healthy skin. The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the product formulation and the integrity of the epidermal barrier. Occlusion, inflammation, and/or other disease processes in the skin may also increase percutaneous absorption. Once absorbed through the skin, topical corticosteroids are metabolized, primarily in the liver, and are then excreted by the kidneys. Some corticosteroids and their metabolites are also excreted in the bile.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of OLUX Foam or clobetasol propionate.
In a 90-day repeat-dose toxicity study in rats, topical administration of clobetasol propionate foam at dose concentrations from 0.001% to 0.1% or from 0.03 to 0.3 mg/kg/day of clobetasol propionate resulted in a toxicity profile consistent with long-term exposure to corticosteroids including adrenal atrophy, histopathological changes in several organs systems indicative of severe immune suppression, and opportunistic fungal and bacterial infections. A no observable adverse effect level (NOAEL) could not be determined in this study. Although the clinical relevance of the findings in animals to humans is not clear, sustained glucocorticoid-related immune suppression may increase the risk of infection and possibly the risk for carcinogenesis.
Topical doses of 0% (foam vehicle), 0.001%, 0.01%, and 0.05% clobetasol propionate foam were evaluated in a 52-week dermal photocarcinogenicity study (40 weeks of treatment followed by 12 weeks of observation) conducted in hairless albino mice with concurrent exposure to low-level ultraviolet radiation. Topical treatment with increasing concentrations of clobetasol propionate foam did not have an adverse effect in this study.
The results of this study suggest that topical treatment with OLUX Foam would not enhance photocarcinogenesis.
Clobetasol propionate was nonmutagenic in 4 different test systems: the Ames test, the mouse lymphoma test, the Saccharomyces cerevisiae gene conversion assay, and the E. coli B WP2 fluctuation test. In the in vivo mouse micronucleus test, a positive finding was observed at 24 hours, but not at 48 hours, following oral administration at a dose of 2,000 mg/kg.
Studies in the rat following subcutaneous administration of clobetasol propionate at dosage levels up to 0.05 mg/kg per day revealed that the females exhibited an increase in the number of resorbed embryos and a decrease in the number of living fetuses at the highest dose.
14 CLINICAL STUDIES
14.1 Scalp Psoriasis
A well-controlled clinical trial evaluated 188 subjects with moderate to severe scalp psoriasis. Subjects were treated twice daily for 2 weeks with one of 4 treatments: OLUX Foam, vehicle foam, a commercially available clobetasol propionate solution (TEMOVATE® Scalp Application), or vehicle solution. The efficacy of OLUX Foam in treating scalp psoriasis at the end of the 2 weeks’ treatment was superior to that of vehicle (foam and solution), and was comparable to that of TEMOVATE Scalp Application (Table 2).
|
|
||
|
OLUX Foam n (%) |
Vehicle Foam n (%) |
|
|
Total number of subjects |
62 |
31 |
|
Subjects with treatment success* |
39 (63) |
1 (3) |
|
Subjects with parameter Clear at endpoint (scalp psoriasis) | ||
|
Scaling - Clear at endpoint |
42 (68) |
3 (10) |
|
Erythema - Clear at endpoint |
27 (44) |
2 (6) |
|
Plaque Thickness - Clear at endpoint |
41 (66) |
3 (10) |
14.2 Non-scalp Psoriasis
Another well-controlled clinical trial evaluated 279 subjects with mild to moderate plaque-type psoriasis (mean body surface area at baseline was 6.7% with a range from 1% to 20%) of non-scalp regions. Subjects were treated twice daily for 2 weeks with OLUX Foam or vehicle foam. The face and intertriginous areas were excluded from treatment. The efficacy of OLUX Foam in treating non-scalp psoriasis at the end of 2 weeks’ treatment was superior to that of vehicle foam (Table 3).
|
|
||
|
OLUX Foam n (%) |
Vehicle Foam n (%) |
|
|
Total number of subjects |
139 |
140 |
|
Subjects with treatment success* |
39 (28) |
4 (3) |
|
Physician's Static Global Assessment -Clear or almost clear at endpoint |
94 (68) |
30 (21) |
|
Scaling - Clear or almost clear at endpoint |
101 (73) |
42 (30) |
|
Erythema - Clear or almost clear at endpoint |
88 (63) |
35 (25) |
|
Plaque Thickness - Clear at endpoint |
44 (32) |
5 (4) |
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
OLUX Foam is a white aerosol foam, supplied as follows:
- 50-g aluminum can NDC: 40076-031-50
- 100-g aluminum can NDC: 40076-031-00
16.2 Storage and Handling
Store at controlled room temperature 68ºF to 77ºF (20ºC to 25ºC).
FLAMMABLE. AVOID FIRE, FLAME, OR SMOKING DURING AND IMMEDIATELY FOLLOWING APPLICATION. Contents under pressure. Do not puncture or incinerate. Do not expose to heat or store at temperatures above 120°F (49°C).
Keep out of reach of children.
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information and Instructions for Use)
Inform patients of the following:
- Avoid use of OLUX Foam on the face, underarms, or groin areas unless directed by the physician.
- Do not occlude the treatment area with bandage or other covering, unless directed by the physician.
- Note that local reactions and skin atrophy are more likely to occur with occlusive use, prolonged use or use of higher potency corticosteroids.
- Discontinue therapy when control is achieved. If no improvement is seen within 2 weeks, contact the physician.
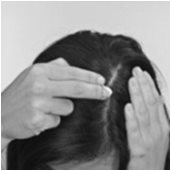
- For proper dispensing of foam, hold the can upside down and depress the actuator. Dispensing directly onto hands is not recommended (unless the hands are the affected area), as the foam will begin to melt immediately upon contact with warm skin.
- Limit treatment to 2 consecutive weeks. Use no more than 50 grams of OLUX Foam per week, or more than 21 capfuls per week.
- Report any signs of local adverse reactions to the physician. Advise patients that local reactions and skin atrophy are more likely to occur with occlusive use or prolonged use.
- Avoid use of OLUX Foam in the diaper area, as diapers or plastic pants may constitute occlusive dressing.
- The product is flammable; avoid heat, flame, and smoking when applying this product.
- Do not use other corticosteroid-containing products without first consulting with the physician.
OLUX is a registered trademark of Stiefel Laboratories, Inc.
For additional information:
1-866-897-5002
or visit
www.olux.com
Manufactured for
Prestium Pharma, Inc.
Newtown, PA 18940
By DPT Laboratories, Ltd.
San Antonio, TX 78215
©2013 Delcor Asset Corporation, an affiliate of Prestium Pharma, Inc.
OLX:XPI
Patient Information
OLUX® (O-lux)
(clobetasol propionate) Foam
|
Important: OLUX Foam is for use on the skin only. Do not get OLUX Foam near or in your eyes, mouth, or vagina. |
What is OLUX Foam?
OLUX Foam is a prescription corticosteroid medicine used on the skin (topical) or scalp to treat adults and children 12 years and older with certain skin conditions that cause red, flaky, and itchy skin.
It is not known if OLUX Foam is safe and effective in children under 12 years of age.
Before using OLUX Foam, tell your healthcare provider about all of your conditions, including if you:
- have had irritation or other skin reaction to a steroid medicine in the past.
- have a skin infection. You may need medicine to treat the skin infection before using OLUX Foam.
- have diabetes.
- have adrenal gland problems.
- have liver problems.
- plan to have surgery.
- are pregnant or plan to become pregnant. It is not known if OLUX Foam will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if OLUX Foam passes into your breast milk. Do not apply OLUX Foam to your chest area if you are breastfeeding a baby. This will help to prevent the baby from accidentally getting OLUX Foam into the baby’s mouth.
Tell your healthcare provider about all the medicine you take including prescription or over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take other corticosteroid medicines by mouth, or injection, or use other products on your skin or scalp that contain corticosteroids.
How should I use OLUX Foam?
- Use OLUX Foam exactly as your healthcare provider tells you to use it.
- This medicine is for use on the skin or scalp only. Do not get OLUX Foam in your eyes, mouth, or vagina.
- Apply OLUX Foam 2 times each day, 1 time in the morning and 1 time in the evening, or as directed by your healthcare provider.
- OLUX Foam should not be used if you have skin thinning (atrophy) at the treatment area.
- Avoid using OLUX Foam on your face, underarms, or groin area.
- Do not bandage or cover your treated area unless your healthcare provider tells you to.
- Do not use OLUX Foam for longer than 2 weeks in a row.
- You should not use more than 50 grams or 21 capfuls of OLUX Foam in 1 week.
- Talk to your healthcare provider if your skin or scalp does not improve after 2 weeks of treatment with OLUX Foam.
- See your healthcare provider regularly to check your symptoms and side effects while using OLUX Foam.
See the “Instructions for Use” at the end of the Patient Information for detailed information about the right way to apply OLUX Foam.
What should I avoid while using OLUX Foam?
OLUX Foam is flammable. Avoid heat, flames, or smoking during and right after you apply it to your skin.
What are the possible side effects of OLUX Foam?
OLUX Foam may cause serious side effects, including:
-
Symptoms of a disorder where the adrenal gland does not make enough of certain hormones (adrenal insufficiency) during treatment or after stopping treatment. Your healthcare provider may do blood tests to check for adrenal insufficiency while you are using OLUX Foam. Tell your healthcare provider if you have any of these persistent symptoms of adrenal insufficiency:
- tiredness that worsens and does not go away, muscle weakness
- loss of appetite
- nausea or vomiting
- dizziness or fainting
- irritability and depression
- weight loss
-
Cushing’s syndrome, when the body is exposed to too much of the hormone cortisol. Your healthcare provider may do tests to check for this. Symptoms can include:
- weight gain, especially around your upper back and midsection
- tiredness and muscle weakness
- roundness of your face (moon face)
- slow healing of cuts, insect bites, and infections
- depression, anxiety, and irritability
- new or worsening high blood pressure
- High blood sugar (hyperglycemia) or diabetes mellitus that has not been diagnosed can happen with treatment. Your healthcare provider may do tests to check you for this.
- Skin problems, including reactions where OLUX Foam is applied, skin infections, and allergic reactions (allergic contact dermatitis). Tell your healthcare provider if you get any new skin problems.
- Effects on growth and weight in children.
The most common side effects of OLUX Foam include burning and skin reactions at the treated site.
These are not all the possible side effects of OLUX Foam.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store OLUX Foam?
- Store the OLUX Foam at room temperature, between 68°F to 77°F (20°C to 25°C).
- Contents are flammable. Keep the can away from fire and heat.
- Do not pierce or burn the can of OLUX Foam. Never throw the can into a fire, even if the can is empty.
Keep OLUX Foam and all medicines out of the reach of children.
General information about the safe and effective use of OLUX Foam.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your healthcare provider or pharmacist for information about OLUX Foam that is written for health professionals. Do not use OLUX Foam for a condition for which it was not prescribed. Do not give OLUX Foam to other people, even if they have the same condition that you have. It may harm them.
What are the ingredients in OLUX Foam?
Active ingredient: clobetasol propionate, USP, 0.05%
Inactive ingredients: cetyl alcohol, citric acid, ethanol (60%), polysorbate 60, potassium citrate, propylene glycol, purified water, and stearyl alcohol pressurized with a hydrocarbon (propane/butane) propellant
Manufactured for:
Prestium Pharma, Inc.,
Newtown, PA 18940
By DPT Laboratories, Ltd., San Antonio, TX 78215
For more information, go to www.olux.com or call 1-866-897-5002.
©2013 Delcor Asset Corporation, an affiliate of Prestium Pharma, Inc.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: October 2014
Instructions for Use
OLUX® (O-lux)
(clobetasol propionate) Foam
Important: OLUX Foam is for use on the skin only. Do not get OLUX Foam near or in your eyes, mouth or vagina.
How should I apply OLUX Foam?
Apply OLUX Foam, 2 times a day, 1 time in the morning and 1 time in the evening, or as directed by your healthcare provider.
Apply only enough to cover the affected areas. OLUX Foam should not be applied to the groin, armpits, or other skin fold areas.
To use OLUX Foam:
How should I store OLUX Foam?
Store the OLUX Foam at room temperature, between 68°F to 77°F (20°C to 25°C).
Contents are flammable. Keep the can away from fire and heat.
Do not pierce or burn the can of OLUX Foam. Never throw the can into a fire, even if the can is empty.
Keep OLUX Foam and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for: Prestium Pharma, Inc.
Newtown, PA 18940
By DPT Laboratories, Ltd. San Antonio, TX 78215
©2013 Delcor Asset Corporation, an affiliate of Prestium Pharma, Inc.
Revised: October 2014
PRINCIPAL DISPLAY PANEL – 0.05%
NDC: 40076-031-00
100 g
Rx only
Olux®
(clobetasol
propionate)
Foam, 0.05%
FOR DERMATOLOGIC USE ONLY.
NOT FOR OPHTHALMIC USE.
Invert can
and then
press firmly
to dispense
Description: Olux® (clobetasol
propionate) Foam, 0.05% mg
clobetasol propionate, USP, per gram in a
thermolabile hydroethanolic foam vehicle
consisting of cetyl alcohol, citric acid,
ethanol (60%), polysorbate 60, potassium
citrate, propylene glycol, purified water,
and stearyl alcohol pressurized with a
hydrocarbon (propane/butane) propellant.
Dosage: Use only as prescribed by your
physician. See package insert for full
prescribing information.
Warning: FLAMMABLE. AVOID FIRE,
FLAME, OR SMOKING DURING AND
IMMEDIATELY FOLLOWING
APPLICATION.
Keep away from eyes. Keep out of reach of
children.
Contents under pressure. Do not puncture or
incinerate container. Do not expose to heat
or stroe at temperatures above 120°
(49°C).
Store at controlled room temperature,
68°-77°F (20°-25°C).
CFC FREE
Manufactured for
Prestium Pharma, Inc.
Newtown, PA 18940
By DPT Laboratories, Ltd.
San Antonio, TX 78215
For additional information:
1-866-897-5002 or visit
www.olux.com
117021-0613
©2013 Delcor Asset Corporation,
an affiliate of Prestium Pharma, Inc.
Olux is a registered trademark of
Stiefel Laboratories, Inc.
| OLUX
clobetasol propionate aerosol, foam |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Prestium Pharma, Inc. (078304674) |