budesonide by Paddock Laboratories, LLC BUDESONIDE capsule
budesonide by
Drug Labeling and Warnings
budesonide by is a Prescription medication manufactured, distributed, or labeled by Paddock Laboratories, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BUDESONIDE EXTENDED-RELEASE CAPSULES safely and effectively. See full prescribing information for BUDESONIDE EXTENDED-RELEASE CAPSULES.
BUDESONIDE extended-release capsules, for oral use
Initial U.S. Approval: 1997RECENT MAJOR CHANGES
- Dosage and Administration (2.1) 09/2018
INDICATIONS AND USAGE
Budesonide extended-release capsules are a corticosteroid indicated for: (1)
- Treatment of mild to moderate active Crohn’s disease involving the ileum and/or the ascending colon, in patients 8 years and older. (1.1)
- Maintenance of clinical remission of mild to moderate Crohn’s disease involving the ileum and/or the ascending colon for up to 3 months in adults. (1.2)
DOSAGE AND ADMINISTRATION
Administration Instructions (2.1): (2)
- Take once daily in the morning.
- Swallow whole. Do not chew or crush.
- For patients unable to swallow an intact capsule, open the capsules and empty the granules onto one tablespoonful of applesauce. Mix and consume the entire contents within 30 minutes. Do not chew or crush. Follow with 8 ounces of water.
- Avoid consumption of grapefruit juice for the duration of therapy.
Recommended Dosage: (2)
Mild to moderate active Crohn’s disease (2.2): (2)
- Adults: 9 mg once daily for up to 8 weeks; repeat 8 week treatment courses for recurring episodes of active disease.
- Pediatrics 8 to 17 years who weigh more than 25 kg: 9 mg once daily for up to 8 weeks, followed by 6 mg once daily in the morning for 2 weeks.
Maintenance of clinical remission of mild to moderate Crohn’s disease (2.3): (2)
- Adults: 6 mg once daily for up to 3 months; taper to complete cessation after 3 months. Continued treatment for more than 3 months has not been shown to provide substantial clinical benefit.
- When switching from oral prednisolone, begin tapering prednisolone concomitantly with initiating budesonide capsules.
Hepatic Impairment: (2)
Consider reducing the dosage to 3 mg once daily in adult patients with moderate hepatic impairment (Child-Pugh Class B). (2.4, 5.1, 8.6) (2)
DOSAGE FORMS AND STRENGTHS
Extended-Release Capsules: 3 mg (3)
CONTRAINDICATIONS
Hypersensitivity to budesonide or any of the ingredients in budesonide extended-release capsules. (4)
WARNINGS AND PRECAUTIONS
- Hypercorticism and Adrenal Axis Suppression: Follow general warnings concerning corticosteroids; pediatrics and patients with hepatic impairment may be at increased risk. (2.4, 5.1, 8.4, 8.6)
- Symptoms of Steroid Withdrawal in Patients Transferred from Other Systemic Corticosteroids: Taper slowly from corticosteroids with high systemic effects; monitor for withdrawal symptoms and unmasking of allergies (rhinitis, eczema). (5.2)
- Increased Risk of Infection, including Serious and Fatal Chicken Pox and Measles: Monitor patients with active or quiescent tuberculosis infection, untreated fungal, bacterial, systemic viral or parasitic infections, or ocular herpes simplex. (5.3)
- Other Corticosteroid Effects: Monitor patients with concomitant conditions where corticosteroids may have unwanted effects (e.g., hypertension, diabetes mellitus). (5.4)
ADVERSE REACTIONS
Most common adverse reactions (≥5%) in adults are: headache, respiratory infection, nausea, back pain, dyspepsia, dizziness, abdominal pain, flatulence, vomiting, fatigue, and pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Perrigo at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
CYP3A4 Inhibitors (e.g., ketoconazole, grapefruit juice): Can increase systemic budesonide concentrations: avoid use. (2.1, 7.1) (7)
USE IN SPECIFIC POPULATIONS
Pregnancy: Based on animal data, may cause fetal harm. (8.1) (8)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Treatment of Mild to Moderate Active Crohn’s Disease
1.2 Maintenance of Clinical Remission of Mild to Moderate Crohn’s Disease
2 DOSAGE AND ADMINISTRATION
2.1 Administration Instructions
2.2 Treatment of Mild to Moderate Active Crohn’s Disease
2.3 Maintenance of Clinical Remission of Mild to Moderate Crohn’s Disease
2.4 Dosage Adjustment in Adult Patients with Hepatic Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypercorticism and Adrenal Axis Suppression
5.2 Symptoms of Steroid Withdrawal in Patients Transferred from Other Systemic Corticosteroids
5.3 Increased Risk of Infection
5.4 Other Corticosteroid Effects
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 CYP3A4 Inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Treatment of Mild to Moderate Active Crohn’s Disease
14.2 Maintenance of Clinical Remission of Mild to Moderate Crohn’s Disease
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Administration Instructions
- Take budesonide extended-release capsules once daily in the morning.
- Swallow budesonide extended-release capsules whole. Do not chew or crush.
- For patients unable to swallow an intact capsule, budesonide extended-release capsules can be opened and administered as follows:
- 1. Place one tablespoonful of applesauce into a clean container (e.g., empty bowl). The applesauce used should not be hot and should be soft enough to be swallowed without chewing.
- 2. Open the capsule(s).
- 3. Carefully empty all the granules inside the capsule(s) on the applesauce.
- 4. Mix the granules with the applesauce.
- 5. Consume the entire contents within 30 minutes of mixing. Do not chew or crush the granules. Do not save the applesauce and granules for future use.
- 6. Follow the applesauce and granules immediately with a glass (8 ounces) of cool water to ensure complete swallowing of the granules.
- Avoid consumption of grapefruit juice for the duration of budesonide extended-release capsule therapy [see Drug Interactions (7.1)].
2.2 Treatment of Mild to Moderate Active Crohn’s Disease
The recommended dosage of budesonide extended-release capsules is:
Adults: 9 mg orally once daily for up to 8 weeks. Repeated 8 week courses of budesonide extended-release capsules can be given for recurring episodes of active disease.
Pediatric patients 8 to 17 years who weigh more than 25 kg: 9 mg orally once daily for up to 8 weeks, followed by 6 mg once daily for 2 weeks.
2.3 Maintenance of Clinical Remission of Mild to Moderate Crohn’s Disease
The recommended dosage in adults, following an 8 week course(s) of treatment for active disease and once the patient’s symptoms are controlled (CDAI less than 150), is budesonide extended-release capsules 6 mg orally once daily for maintenance of clinical remission up to 3 months. If symptom control is still maintained at 3 months an attempt to taper to complete cessation is recommended. Continued treatment with budesonide extended-release capsules 6 mg for more than 3 months has not been shown to provide substantial clinical benefit.
Patients with mild to moderate active Crohn’s disease involving the ileum and/or ascending colon have been switched from oral prednisolone to budesonide extended-release capsules with no reported episodes of adrenal insufficiency. Since prednisolone should not be stopped abruptly, tapering should begin concomitantly with initiating budesonide extended-release capsules treatment.
2.4 Dosage Adjustment in Adult Patients with Hepatic Impairment
Consider reducing the dosage of budesonide extended-release capsules to 3 mg once daily for adult patients with moderate hepatic impairment (Child-Pugh Class B). Avoid use in patients with severe hepatic impairment (Child-Pugh Class C) [see Warnings and Precautions (5.1), Use in Specific Populations (8.6)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypercorticism and Adrenal Axis Suppression
When corticosteroids are used chronically, systemic effects such as hypercorticism and adrenal axis suppression may occur. Corticosteroids can reduce the response of the hypothalamus-pituitary-adrenal (HPA) axis to stress. In situations where patients are subject to surgery or other stress situations, supplementation with a systemic corticosteroid is recommended.
Since budesonide extended-release capsules contain a corticosteroid, general warnings concerning corticosteroids should be followed [see Warnings and Precautions (5.2), (5.3), (5.4)].
Pediatric patients with Crohn’s disease have a slightly higher systemic exposure of budesonide and increased cortisol suppression than adults with Crohn’s disease [see Use in Specific Populations (8.4), Clinical Pharmacology (12.2)]. Patients with moderate to severe hepatic impairment (Child-Pugh Class B and C respectively) could be at an increased risk of hypercorticism and adrenal axis suppression due to an increased systemic exposure of oral budesonide. Avoid use in patients with severe hepatic impairment (Child-Pugh Class C). Monitor for increased signs and/or symptoms of hypercorticism and consider reducing the dosage in patients with moderate hepatic impairment (Child-Pugh Class B) [see Dosage and Administration (2.4), Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
5.2 Symptoms of Steroid Withdrawal in Patients Transferred from Other Systemic Corticosteroids
Monitor patients who are transferred from corticosteroid treatment with high systemic effects to corticosteroids with lower systemic availability, such as budesonide extended-release capsules, since symptoms attributed to withdrawal of steroid therapy, including those of acute adrenal axis suppression or benign intracranial hypertension, may develop. Adrenocortical function monitoring may be required in these patients and the dose of corticosteroid treatment with high systemic effects should be reduced cautiously.
Replacement of systemic corticosteroids with budesonide extended-release capsules may unmask allergies (e.g., rhinitis and eczema), which were previously controlled by the systemic drug.
5.3 Increased Risk of Infection
Patients who are on drugs that suppress the immune system are more susceptible to infection than healthy individuals. Chicken pox and measles, for example, can have a more serious or even fatal course in susceptible patients or patients on immunosuppressant doses of corticosteroids. In patients who have not had these diseases, particular care should be taken to avoid exposure.
How the dose, route and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed, therapy with varicella zoster immune globulin (VZIG) or pooled intravenous immunoglobulin (IVIG), as appropriate, may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See prescribing information for VZIG and IG). If chicken pox develops, treatment with antiviral agents may be considered.
Corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculosis infection, untreated fungal, bacterial, systemic viral or parasitic infections, or ocular herpes simplex.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
- Hypercorticism and adrenal axis suppression [see Warnings and Precautions (5.1)]
- Symptoms of steroid withdrawal in those patients transferred from other systemic corticosteroids [see Warnings and Precautions (5.2)]
- Increased risk of infection [see Warnings and Precautions (5.3)]
- Other corticosteroid effects [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults
The data described below reflect exposure to budesonide extended-release capsules in 520 patients with Crohn’s disease, including 520 exposed to 9 mg per day (total daily dose) for 8 weeks and 145 exposed to 6 mg per day for one year in placebo controlled clinical trials. Of the 520 patients, 38% were males and the age range was 17 to 74 years.
Treatment of Mild to Moderate Active Crohn’s Disease
The safety of budesonide extended-release capsules was evaluated in 651 adult patients in five clinical trials of 8 weeks duration in patients with active mild to moderate Crohn’s disease. The most common adverse reactions, occurring in greater than or equal to 5% of the patients, are listed in Table 1.
Table 1: Common Adverse Reactions1 in 8-Week Treatment Clinical Trials
Adverse Reaction
Budesonide Extended-Release Capsules 9 mg
n=520
Number (%)
Placebo
n=107
Number (%)
Prednisolone2
40 mg
n=145
Number (%)
Comparator3
n=88
Number (%)
Headache
107 (21)
19 (18)
31 (21)
11 (13)
Respiratory Infection
55 (11)
7 (7)
20 (14)
5 (6)
Nausea
57 (11)
10 (9)
18 (12)
7 (8)
Back Pain
36 (7)
10 (9)
17 (12)
5 (6)
Dyspepsia
31 (6)
4 (4)
17 (12)
3 (3)
Dizziness
38 (7)
5 (5)
18 (12)
5 (6)
Abdominal Pain
32 (6)
18 (17)
6 (4)
10 (11)
Flatulence
30 (6)
6 (6)
12 (8)
5 (6)
Vomiting
29 (6)
6 (6)
6 (4)
6 (7)
Fatigue
25 (5)
8 (7)
11 (8)
0 (0)
Pain
24 (5)
8 (7)
17 (12)
2 (2)
1. Occurring in greater than or equal to 5% of the patients in any treated group.
2. Prednisolone tapering scheme: either 40 mg in week 1 to 2, thereafter tapering with 5 mg per week; or 40 mg in week 1 to 2, 30 mg in week 3 to 4, thereafter tapering with 5 mg per week.
3. This drug is not approved for the treatment of Crohn’s disease in the United States.
The incidence of signs and symptoms of hypercorticism reported by active questioning of patients in 4 of the 5 short-term clinical trials are displayed in Table 2.
Table 2: Summary and Incidence of Signs/Symptoms of Hypercorticism in 8-Week Treatment Clinical Trials
Signs/Symptom
Budesonide Extended-Release Capsules
9 mg
n=427
Number (%)
Placebo
n=107
Number (%)
Prednisolone1
40 mg
n=145
Number (%)
Total
145 (34%)
29 (27%)
69 (48%)
Acne
63 (15)
14 (13)
33 (23)2
Bruising Easily
63 (15)
12 (11)
13 (9)
Moon Face
46 (11)
4 (4)
53 (37)2
Swollen Ankles
32 (7)
6 (6)
13 (9)
Hirsutism3
22 (5)
2 (2)
5 (3)
Buffalo Hump
6 (1)
2 (2)
5 (3)
Skin Striae
4 (1)
2 (2)
0 (0)
1. Prednisolone tapering scheme: either 40 mg in week 1-2, there after tapering with 5 mg/week; or 40 mg in week 1 to 2, 30 mg in week 3 to 4, thereafter tapering with 5 mg/week.
2. Statistically significantly different from budesonide extended-release capsules 9 mg.
3. Including hair growth increased, local and hair growth increased, general.
Maintenance of Clinical Remission of Mild to Moderate Crohn’s Disease
The safety of budesonide extended-release capsules was evaluated in 233 adult patients in four long-term clinical trials (52 weeks) of maintenance of clinical remission in patients with mild to moderate Crohn’s disease. A total of 145 patients were treated with budesonide extended-release capsules 6 mg once daily.
The adverse reaction profile of budesonide extended-release capsules 6 mg once daily in maintenance of Crohn’s disease was similar to that of short-term treatment with budesonide extended-release capsules 9 mg once daily in active Crohn’s disease. In the long-term clinical trials, the following adverse reactions occurred in greater than or equal to 5% and are not listed in Table 1: diarrhea (10%); sinusitis (8%); infection viral (6%); and arthralgia (5%).
Signs/symptoms of hypercorticism reported by active questioning of patients in the long-term maintenance clinical trials are displayed in Table 3.
Table 3: Summary and Incidence of Signs/Symptoms of Hypercorticism in Long-Term Clinical Trials
Signs/Symptom
Budesonide Extended-Release Capsules
3 mg
n=88
Number (%)
Budesonide Extended-Release Capsules
6 mg
n=145
Number (%)
Placebo
n=143
Number (%)
Bruising Easily
4 (5)
15 (10)
5 (4)
Acne
4 (5)
14 (10)
3 (2)
Moon Face
3 (3)
6 (4)
0
Hirsutism
2 (2)
5 (3)
1(1)
Swollen Ankles
2 (2)
3 (2)
3 (2)
Buffalo Hump
1 (1)
1 (1)
0
Skin Striae
2 (2)
0
0
The incidence of signs/symptoms of hypercorticism as described above in long-term maintenance clinical trials was similar to that seen in the short-term treatment clinical trials.
Less Common Adverse Reactions in Treatment and Maintenance Clinical Trials
Less common adverse reactions (less than 5%), occurring in adult patients treated with budesonide extended-release capsules 9 mg (total daily dose) in short-term treatment clinical studies and/or budesonide extended-release capsules 6 mg (total daily dose) in long-term maintenance clinical trials, with an incidence are listed below by system organ class:
Cardiac disorders: palpitation, tachycardia
Eye disorders: eye abnormality, vision abnormal
General disorders and administration site conditions: asthenia, chest pain, dependent edema, face edema, flu-like disorder, malaise, fever
Gastrointestinal disorders: anus disorder, enteritis, epigastric pain, gastrointestinal fistula, glossitis, hemorrhoids, intestinal obstruction, tongue edema, tooth disorder
Infections and infestations: Ear infection - not otherwise specified, bronchitis, abscess, rhinitis, urinary tract infection, thrush
Investigations: weight increased
Metabolism and nutrition disorders: appetite increased
Musculoskeletal and connective tissue disorders: arthritis, cramps, myalgia
Nervous system disorders: hyperkinesia, paresthesia, tremor, vertigo, somnolence, amnesia
Psychiatric disorders: agitation, confusion, insomnia, nervousness, sleep disorder
Renal and urinary disorders: dysuria, micturition frequency, nocturia
Reproductive system and breast disorders: intermenstrual bleeding, menstrual disorder
Respiratory, thoracic and mediastinal disorders: dyspnea, pharynx disorder
Skin and subcutaneous tissue disorders: alopecia, dermatitis, eczema, skin disorder, sweating increased, purpura
Vascular disorders: flushing, hypertension
Bone Mineral Density
A randomized, open, parallel-group multicenter safety clinical trial specifically compared the effect of budesonide extended-release capsules (less than 9 mg per day) and prednisolone (less than 40 mg per day) on bone mineral density over 2 years when used at doses adjusted to disease severity. Bone mineral density decreased significantly less with budesonide extended-release capsules than with prednisolone in steroid-naïve patients, whereas no difference could be detected between treatment groups for steroid-dependent patients and previous steroid users. The incidence of symptoms associated with hypercorticism was significantly higher with prednisolone treatment.
Clinical Laboratory Test Findings
The following potentially clinically significant laboratory changes in clinical trials, irrespective of relationship to budesonide extended-release capsules, were reported in greater than or equal to 1% of patients: hypokalemia, leukocytosis, anemia, hematuria, pyuria, erythrocyte sedimentation rate increased, alkaline phosphatase increased, atypical neutrophils, c-reactive protein increased and adrenal insufficiency.
Pediatrics -- Treatment of Mild to Moderate Active Crohn’s Disease
Adverse reactions reported in pediatric patients 8 to 17 years of age, who weigh more than 25 kg, were similar to those reactions described above in adult patients.
6.2 Postmarketing Experience
The following adverse reactions have been reported during post-approval use of budesonide extended-release capsules. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: Anaphylactic reactions
Nervous System Disorders: Benign intracranial hypertension
Psychiatric Disorders: Mood swings
-
7 DRUG INTERACTIONS
7.1 CYP3A4 Inhibitors
Budesonide is a substrate for CYP3A4. Avoid use with CYP3A4 inhibitors. Concomitant oral administration of a strong CYP3A4 inhibitor (ketoconazole) caused an eight-fold increase of the systemic exposure to oral budesonide. Inhibitors of CYP3A4 (e.g., ketoconazole, itraconazole, ritonavir, indinavir, saquinavir, erythromycin, and cyclosporine) can increase systemic budesonide concentrations [see Clinical Pharmacology (12.3)].
Grapefruit Juice
Avoid ingestion of grapefruit juice with budesonide. Intake of grapefruit juice which inhibits CYP3A4 activity with budesonide can increase the systemic exposure for budesonide [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited published studies report on the use of budesonide in pregnant women; however, the data are insufficient to inform a drug-associated risk for major birth defects and miscarriage. There are clinical considerations [see Clinical Considerations]. In animal reproduction studies with pregnant rats and rabbits, administration of subcutaneous budesonide during organogenesis at doses approximately 0.5 times or 0.05 times, respectively, the maximum recommended human dose, resulted in increased fetal loss, decreased pup weights, and skeletal abnormalities. Maternal toxicity was observed in both rats and rabbits at these dose levels [see Data]. Based on animal data, advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage of the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Some published epidemiological studies show an association of adverse pregnancy outcomes in women with Crohn’s disease, including preterm birth and low birth weight infants, during periods of increased disease activity (including increased stool frequency and abdominal pain). Pregnant women with Crohn’s disease should be counseled regarding the importance of controlling disease.
Fetal/Neonatal adverse reactions
Hypoadrenalism may occur in infants born of mothers receiving corticosteroids during pregnancy. Infants should be carefully observed for signs of hypoadrenalism, such as poor feeding, irritability, weakness, and vomiting, and managed accordingly [see Warnings and Precautions (5.1)].
Data
Animal Data
Budesonide was teratogenic and embryolethal in rabbits and rats.
In an embryo-fetal development study in pregnant rats dosed subcutaneously with budesonide during the period of organogenesis from gestation days 6-15 there were effects on fetal development and survival at subcutaneous doses up to approximately 500 mcg/kg in rats (approximately 0.5 times the maximum recommended human dose on a body surface area basis). In an embryo-fetal development study in pregnant rabbits dosed during the period of organogenesis from gestation days 6-18, there was an increase in maternal abortion, and effects on fetal development and reduction in litter weights at subcutaneous doses up to approximately 25 mcg/kg in rabbits (approximately 0.05 times the maximum recommended human dose on a body surface area basis). Maternal toxicity, including reduction in body weight gain, was observed at subcutaneous doses of 5 mcg/kg in rabbits (approximately 0.01 times the maximum recommended human dose on a body surface area basis) and 500 mcg/kg in rats (approximately 0.5 times the maximum recommended human dose on a body surface area basis).
In a peri-and post-natal development study, rats dosed subcutaneously with budesonide during the period of Day 15 post coitum to Day 21 postpartum, budesonide had no effects on delivery but did have an effect on growth and development of offspring. In addition, offspring survival was reduced and surviving offspring had decreased mean body weights at birth and during lactation at exposures 0.02 times the MRHD (on a mg/m2 basis at maternal subcutaneous doses of 20 mcg/kg/day and higher). These findings occurred in the presence of maternal toxicity.
8.2 Lactation
Risk Summary
Lactation studies have not been conducted with oral budesonide, including budesonide extended-release capsules, and no information is available on the effects of the drug on the breastfed infant or the effects of the drug on milk production. One published study reports that budesonide is present in human milk following maternal inhalation of budesonide [see Data]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for budesonide extended-release capsules and any potential adverse effects on the breastfed infant from budesonide extended-release capsules, or from the underlying maternal condition.
Data
One published study reports that budesonide is present in human milk following maternal inhalation of budesonide which resulted in infant doses approximately 0.3% to 1% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 0.4 and 0.5. Budesonide plasma concentrations were not detected and no adverse events were noted in the breastfed infants following maternal use of inhaled budesonide. The recommended daily dose of budesonide extended-release capsules is higher (up to 9 mg daily) compared with inhaled budesonide (up to 800 mcg daily) given to mothers in the above described study.
The maximum budesonide plasma concentration following a 9 mg daily dose (in both single- and repeated-dose pharmacokinetic studies) of oral budesonide is approximately 2.15 to 4.31 ng/mL which is up to 10 times higher than the 0.43 to 0.86 ng/mL for a 800 mcg daily dose of inhaled budesonide at steady state in the above inhalation study. Assuming the coefficient of extrapolation between the inhaled and oral doses is constant across all dose levels, at therapeutic doses of budesonide extended-release capsules, budesonide exposure to the nursing child may be up to 10 times higher than that by budesonide inhalation.
8.4 Pediatric Use
The safety and effectiveness of budesonide extended-release capsules have been established in pediatric patients 8 to 17 years of age who weigh more than 25 kg for the treatment of mild to moderate active Crohn’s disease involving the ileum and/or the ascending colon. Use of budesonide extended-release capsules in this age group is supported by evidence from adequate and well controlled studies of budesonide extended-release capsules in adults, with additional data from 2 clinical studies in 149 pediatric patients treated up to 8 weeks and one pharmacokinetic study in 8 pediatric patients [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.1)].
The observed safety profile of budesonide extended-release capsules in pediatric patients is consistent with its known safety profile in adults and no new safety concerns were identified [see Adverse Reactions (6.1)].
The safety and effectiveness of budesonide extended-release capsules have not been established in pediatric patients less than 8 years of age for the treatment of mild to moderate active Crohn’s disease involving the ileum and/or the ascending colon.
The safety and effectiveness of budesonide extended-release capsules have not been established in pediatric patients for the maintenance of clinical remission of mild to moderate Crohn’s disease. An open-label study to evaluate the safety and tolerability of budesonide extended-release capsules as maintenance treatment in pediatric patients aged 5 to 17 years was conducted, and did not establish the safety and efficacy of maintenance of clinical remission.
Systemic corticosteroids, including budesonide extended-release capsules, may cause a reduction of growth velocity in pediatric patients. Pediatric patients with Crohn’s disease have a 17% higher mean systemic exposure and cortisol suppression than adults with Crohn’s disease [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.2)].
8.5 Geriatric Use
Clinical studies of budesonide extended-release capsules did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. Of the 651 patients treated with budesonide extended-release capsules in clinical studies, 17 (3%) were greater than or equal to 65 years of age and none were greater than 74 years of age. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
Patients with moderate to severe hepatic impairment (Child-Pugh Class B and C, respectively) could be at an increased risk of hypercorticism and adrenal axis suppression due to an increased systemic exposure to budesonide [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)]. Avoid use in patients with severe hepatic impairment (Child-Pugh Class C). Monitor for increased signs and/or symptoms of hypercorticism and consider dosage reduction in patients with moderate hepatic impairment (Child-Pugh Class B) [see Dosage and Administration (2.4)]. No dosage adjustment is needed in patients with mild hepatic impairment (Child-Pugh Class A).
-
10 OVERDOSAGE
Reports of acute toxicity and/or death following overdosage of glucocorticoids are rare. Treatment consists of immediate gastric lavage or emesis followed by supportive and symptomatic therapy.
If corticosteroids are used at excessive doses for prolonged periods, systemic corticosteroid effects such as hypercorticism and adrenal axis suppression may occur. For chronic overdosage in the case of severe disease requiring continuous steroid therapy, the dosage may be reduced temporarily.
Single oral doses of 200 and 400 mg/kg were lethal in female and male mice, respectively. The signs of acute toxicity were decreased motor activity, piloerection and generalized edema.
-
11 DESCRIPTION
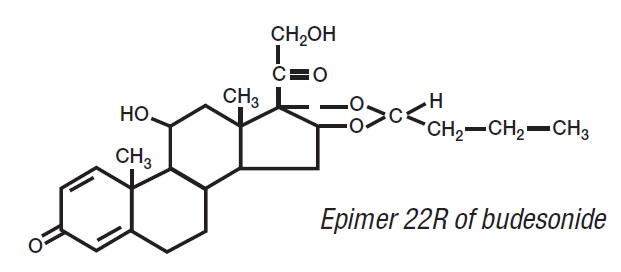
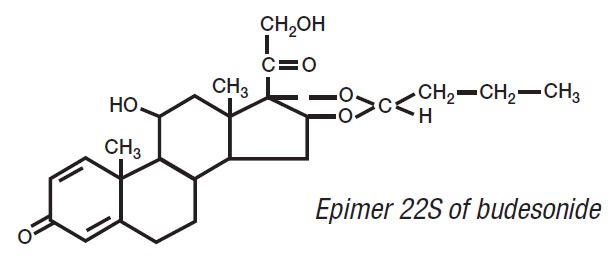
Budesonide, the active ingredient of budesonide extended-release capsules, is a synthetic corticosteroid. Budesonide is designated chemically as (RS)-11β, 16α, 17,21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with butyraldehyde. Budesonide is provided as a mixture of two epimers (22R and 22S). The empirical formula of budesonide is C25H34O6 and its molecular weight is 430.5. Its structural formula is:
Budesonide is a white to off-white, tasteless, odorless powder that is practically insoluble in water and heptane, sparingly soluble in ethanol, and freely soluble in chloroform. Its partition coefficient between octanol and water at pH 5 is 1.6 x 103 ionic strength 0.01.
Budesonide extended-release capsules are formulated as hard gelatin capsules filled with enteric-coated granules that dissolve at pH greater than 5.5. Each capsule for oral administration contains 3 mg of micronized budesonide with the following inactive ingredients: ethylcellulose, acetyltributyl citrate, methacrylic acid copolymer type C, triethyl citrate, antifoam M, polysorbate 80, talc, and sugar spheres. The capsule shells have the following inactive ingredients: gelatin, iron oxide, and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Budesonide is an anti-inflammatory corticosteroid and has a high glucocorticoid effect and a weak mineralocorticoid effect, and the affinity of budesonide to glucocorticoid receptors, which reflects the intrinsic potency of the drug, is about 200-fold that of cortisol and 15-fold that of prednisolone.
12.2 Pharmacodynamics
Treatment with glucocorticoids, including budesonide extended-release capsules is associated with a suppression of endogenous cortisol concentrations and an impairment of the hypothalamus-pituitary-adrenal (HPA) axis function. There was a positive correlation between the percent (%) reduction of AUC0-24 of plasma cortisol and systemic exposure to budesonide both in pediatric and adult patients.
Adults
Plasma cortisol suppression was compared following five days’ administration of budesonide extended-release capsules and prednisolone in a crossover study in healthy volunteers. The mean decrease in the area under the plasma cortisol concentration-time curve over 24 hour (AUC0-24) was greater (78%) with prednisolone 20 mg per day compared to 45% with budesonide extended-release capsules 9 mg per day.
Pediatrics
The effect of budesonide on endogenous cortisol concentrations was compared between pediatrics (n=8, aged 9 to 14 years) and adults (n=6) with active Crohn’s disease following administration of budesonide extended-release capsules 9 mg once daily for 7 days. Compared to baseline values before treatment, the mean decrease in the AUC0-24 of cortisol was 64% (±18%) in pediatrics and 50% (±27%) in adults after budesonide extended-release capsules treatment [see Warnings and Precautions (5.1), Adverse Reactions (6.1) and Use in Specific Populations (8.4)].
The responses to adrenocorticotropin challenge (i.e., ACTH stimulation test) was studied in pediatric patients aged 8 to 17 years, with mild to moderate active Crohn’s disease in randomized, double-blind, active control study [see Clinical Studies (14.1)]. After 8 weeks of treatment with 9 mg once daily budesonide extended-release capsules or with prednisolone, administered at tapering doses starting from 1 mg/kg, the proportion of patients with normal response to the ACTH challenge was 6% in the budesonide group compared to none in the prednisolone group; the proportion of patients with morning p-cortisol of greater than 5 mcg/dL was 50% in the budesonide group compared to 22% in the prednisolone group. The mean morning p-cortisol was 6.3 mcg/dL in the budesonide group and 2.6 mcg/dL in the prednisolone group (Table 4).
Table 4. Proportion of Pediatric Patients 8 to 17 years old with Peak Endogenous Cortisol Levels (above 18 mcg/dL) after ACTH Stimulation and Normal Response* to ACTH Challenge Following Administration of Budesonide Extended-Release Capsules or Prednisolone for 8 weeks
Budesonide
Prednisolone
Peak plasma cortisol above 18 mcg/dL
At baseline
91% (20/22)
91% (21/23)
At week 8
25% (4/16)
0% (0/18)
Normal response* to ACTH challenge
At baseline
73% (16/22)
78% (18/23)
At week 8
6% (1/16)
0% (0/18)
*The normal response to ACTH challenge included 3 criteria, as defined in the cosyntropin label:
1) morning cortisol level above 5 mcg/dL; 2) increase in cortisol level by at least 7 mcg/dL above the morning (pre-challenge) level following ACTH challenge; and cortisol level of above 18 mcg/dL following ACTH challenge. Cortisol concentration was measured at 30 min after intravenous or intramuscular injection of 0.25 mg cosyntropin at baseline and at week 8 after treatment.
12.3 Pharmacokinetics
Absorption
Following administration of budesonide extended-release capsules, the time to peak concentration varied in individual patients between 30 and 600 minutes. Mean oral bioavailability of budesonide ranged from 9% to 21% both in patients and in healthy subjects, demonstrating a high first-pass elimination of the drug.
Budesonide pharmacokinetics were dose-proportional following repeated administration in the dose range of 3 to 15 mg. No accumulation of budesonide was observed following repeated dosing.
Following oral administration of a single dose of 9 mg budesonide extended-release capsules in healthy subjects under fasting condition, the mean peak plasma concentration (Cmax) and the area under the plasma concentration time curve (AUC) for budesonide were 1.50 ± 0.79 ng/mL and 14.13 ± 7.33 nghr/mL, respectively. The time to peak concentration (Tmax) varied between 2 and 8 hours with a median value of 3.5 hours. In a different study, following oral administration of 9 mg budesonide extended-release capsules for five days in healthy subjects, the mean Cmax and the steady state AUC for budesonide were 2.28 ± 0.77 ng/mL and 15.93 ± 6.29 nghr/mL, respectively.
Following administration of 9 mg budesonide extended-release capsules once daily in patients with active Crohn’s disease, the mean Cmax and AUC were 1.72 ± 0.90 ng/mL and 15.07 ± 8.52 nghr/mL, respectively.
Concomitant administration of a high-fat meal delayed the Tmax of budesonide from budesonide extended-release capsules by 2.3 hours but did not significantly affect the AUC in healthy subjects. The Cmax and AUC to budesonide was similar when single dose of budesonide extended-release capsules (9 mg) were administered after opening capsule and sprinkling granules on applesauce versus as intact capsules in the fasted state (N=24) in healthy subjects.
Distribution
The mean volume of distribution (Vss) of budesonide varied between 2.2 and 3.9 L/kg in healthy subjects and in patients. Plasma protein binding was estimated to be 85% to 90% in the concentration range 0.43 to 99.02 ng/mL, independent of gender. The erythrocyte/plasma partition ratio at clinically relevant concentrations was about 0.8.
Elimination
Budesonide had a plasma clearance, 0.9 to 1.8 L/min in healthy adults. Mean plasma clearance after intravenous administration of budesonide in patients with Crohn’s disease was 1.0 L/min. These plasma clearance values approached the estimated liver blood flow, and, accordingly, suggest that budesonide is a high hepatic clearance drug. The plasma elimination half-life, after administration of intravenous doses ranged between 2 and 3.6 hours, and did not differ between healthy adults and patients with Crohn’s disease.
Metabolism
Following absorption, budesonide is subject to high first pass metabolism (80% to 90%). In vitro experiments in human liver microsomes demonstrated that budesonide is rapidly and extensively biotransformed, mainly by CYP3A4, to its 2 major metabolites, 6β-hydroxy budesonide and 16α-hydroxy prednisolone. The corticosteroid activity of these metabolites was negligible (less than 1/100) in relation to that of the parent compound. In vivo investigations with intravenous doses in healthy subjects were in agreement with the in vitro findings.
Excretion
Budesonide was excreted in urine and feces in the form of metabolites. After oral as well as intravenous administration of micronized [3H]-budesonide, approximately 60% of the recovered radioactivity was found in urine. The major metabolites, including 6β-hydroxy budesonide and 16α-hydroxy prednisolone, are mainly renally excreted, intact or in conjugated forms. No unchanged budesonide was detected in urine.
Specific Populations
Age: Pediatric Population (8 years and older)
The pharmacokinetics of budesonide were investigated in pediatric patients aged 9 to 14 years (n=8) after oral administration of budesonide extended-release capsules and intravenous administration of budesonide. Following administration of 9 mg budesonide extended-release capsules once daily for 7 days, the median time to peak plasma concentration of budesonide was 5 hours and the mean peak plasma concentration was 2.58 ± 1.51 ng/mL. The mean AUC was 17.78 ± 5.25 nghr/mL and 17% higher than that in adult patients with Crohn’s disease in the same study. The mean absolute oral availability was 9.2% (3 to 17%; n=4) in pediatric patients.
After single dose administration of intravenous budesonide (n=4), the mean volume of distribution (Vss) was 2.2 ± 0.4 L/kg and mean clearance was 0.81 ± 0.2 L/min. The mean elimination half-life was 1.9 hours in pediatric patients. The body-weight normalized clearance in pediatric patients was 20.5 mL/min/kg in comparison to 15.9 mL/min/kg in adult patients after intravenous administration [see Warnings and Precautions (5.1), Use in Specific Population (8.4)].
Hepatic Impairment
In patients with mild (Child-Pugh Class A, n=4) or moderate (Child-Pugh Class B, n=4) hepatic impairment, budesonide 4 mg was administered orally as a single dose. The patients with moderate hepatic impairment had a 3.5-fold higher AUC compared to the healthy subjects with normal hepatic function while the patients with mild hepatic impairment had an approximately 1.4-fold higher AUC. The Cmax values demonstrated similar increases [see Dosage and Administration (2.4), Warnings and Precautions (5.1)]. The increased systemic exposure in patients with mild hepatic impairment was not considered to be clinically relevant. Patients with severe liver impairment (Child-Pugh Class C) were not studied.
Drug Interaction Studies
Budesonide is metabolized via CYP3A4. Potent inhibitors of CYP3A4 can increase the plasma concentrations of budesonide several-fold. Conversely, induction of CYP3A4 potentially could result in the lowering of budesonide plasma concentrations.
Effects of Other Drugs on Budesonide
Ketoconazole
In an open, non-randomized, cross-over study, 6 healthy subjects were given budesonide 10 mg as a single dose, either alone or concomitantly with the last ketoconazole dose of 3 days treatment with ketoconazole 100 mg twice daily. Co-administration of ketoconazole resulted in an eight-fold increase in AUC of budesonide, compared to budesonide alone [see Drug Interactions (7.1)].
Grapefruit Juice
In an open, randomized, cross-over study, 8 healthy subjects were given budesonide capsules 3 mg, either alone, or concomitantly with 600 mL concentrated grapefruit juice (which inhibits CYP3A4 activity predominantly in the intestinal mucosa), on the last of 4 daily administrations. Concomitant administration of grapefruit juice resulted in a 2-fold increase of the bioavailability of budesonide compared to budesonide alone [see Drug Interactions (7.1)].
Oral Contraceptives (CYP3A4 Substrates)
In a parallel study, the pharmacokinetics of budesonide were not significantly different between healthy female subjects who received oral contraceptives containing desogestrel 0.15 mg and ethinyl estradiol 30 μg and healthy female subjects who did not receive oral contraceptives. Budesonide 4.5 mg once daily (one-half the recommended dose) for one week did not affect the plasma concentrations of ethinyl estradiol, a CYP3A4 substrate. The effect of budesonide 9 mg once daily on the plasma concentrations of ethinyl estradiol was not studied.
Omeprazole
In a study in 11 healthy subjects, performed in a double-blind, randomized, placebo controlled manner, the effect of 5 to 6 days treatment with omeprazole 20 mg once daily on the pharmacokinetics of budesonide administered as budesonide capsules 9 mg as a single dose was investigated. Omeprazole 20 mg once daily did not affect the absorption or pharmacokinetics of budesonide.
Cimetidine
In an open, non-randomized, cross-over study, the potential effect of cimetidine on the pharmacokinetics of budesonide was studied. Six healthy subjects received cimetidine 1 gram daily (200 mg with meals and 400 mg at night) for 2 separate 3-day periods. Budesonide 4 mg was administered either alone or on the last day of one of the cimetidine treatment periods. Co-administration of cimetidine resulted in a 52% and 31% increase in the budesonide peak plasma concentration and the AUC of budesonide, respectively.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with budesonide were conducted in rats and mice. In a two-year study in Sprague-Dawley rats, budesonide caused a statistically significant increase in the incidence of gliomas in male rats at an oral dose of 50 mcg/kg (approximately 0.05 times the maximum recommended human dose on a body surface area basis). In addition, there were increased incidences of primary hepatocellular tumors in male rats at 25 mcg/kg (approximately 0.023 times the maximum recommended human dose on a body surface area basis) and above. No tumorigenicity was seen in female rats at oral doses up to 50 mcg/kg (approximately 0.05 times the maximum recommended human dose on a body surface area basis). In an additional two-year study in male Sprague-Dawley rats, budesonide caused no gliomas at an oral dose of 50 mcg/kg (approximately 0.05 times the maximum recommended human dose on a body surface area basis). However, it caused a statistically significant increase in the incidence of hepatocellular tumors at an oral dose of 50 mcg/kg (approximately 0.05 times the maximum recommended human dose on a body surface area basis). The concurrent reference corticosteroids (prednisolone and triamcinolone acetonide) showed similar findings. In a 91-week study in mice, budesonide caused no treatment-related carcinogenicity at oral doses up to 200 mcg/kg (approximately 0.1 times the maximum recommended human dose on a body surface area basis).
Budesonide was not genotoxic in the Ames test, the mouse lymphoma cell forward gene mutation (TK +/-) test, the human lymphocyte chromosome aberration test, the Drosophila melanogaster sex-linked recessive lethality test, the rat hepatocyte UDS test and the mouse micronucleus test.
In rats, budesonide had no effect on fertility at subcutaneous doses up to 80 mcg/kg (approximately 0.07 times the maximum recommended human dose on a body surface area basis). However, it caused a decrease in prenatal viability and viability in pups at birth and during lactation, along with a decrease in maternal body-weight gain, at subcutaneous doses of 20 mcg/kg (approximately 0.02 times the maximum recommended human dose on a body surface area basis) and above. No such effects were noted at 5 mcg/kg (approximately 0.005 times the maximum recommended human dose on a body surface area basis).
-
14 CLINICAL STUDIES
14.1 Treatment of Mild to Moderate Active Crohn’s Disease
Adults
The efficacy of budesonide extended-release capsules were evaluated in 994 patients with mild to moderate active Crohn’s disease of the ileum and/or ascending colon in 5 randomized and double-blind studies of 8 weeks duration. The study patients ranged in age from 17 to 85 (mean 35), 40% were male and 97% were white. The Crohn’s Disease Activity Index (CDAI) was the main clinical assessment used for determining efficacy in these 5 studies.1 The CDAI is a validated index based on subjective aspects rated by the patient (frequency of liquid or very soft stools, abdominal pain rating and general well-being) and objective observations (number of extraintestinal symptoms, need for antidiarrheal drugs, presence of abdominal mass, body weight and hematocrit). Clinical improvement, defined as a CDAI score of less than or equal to 150 assessed after 8 weeks of treatment, was the primary efficacy variable in these 5 comparative efficacy studies of budesonide extended-release capsules. Safety assessments in these studies included monitoring of adverse reactions. A checklist of potential symptoms of hypercorticism was used.
One study (Study 1) compared the efficacy of budesonide extended-release capsules 9 mg daily in the morning to a comparator. At baseline, the median CDAI was 272. Budesonide extended-release capsules 9 mg daily resulted in a significantly higher clinical improvement rate at Week 8 than the comparator. See Table 5.
Table 5: Clinical Improvement Rates (CDAI less than or equal to 150) After 8 weeks of Treatment
Clinical Study
Budesonide Extended-Release Capsules
9 mg Daily
Budesonide Extended-Release Capsules
4.5 mg Twice Daily
Comparator3
Placebo
Prednisolone
1
62/91 (69%)1
37/83 (45%)
2
31/61 (51%)2
13/64 (20%)
3
38/79 (48%)
41/78 (53%)
13/40 (33%)
4
35/58 (60%)
25/60 (42%)
35/58 (60%)
5
45/86 (52%)
56/85 (65%)
1. p=0.0004 compared to comparator.
2. p=0.001 compared to placebo.
3. This drug is not approved for the treatment of Crohn’s disease in the United States.
Two placebo-controlled clinical trials (Studies 2 and 3) were conducted. Study 2 involved 258 patients and tested the effects of graded doses of budesonide extended-release capsules (1.5 mg twice daily, 4.5 mg twice daily, or 7.5 mg twice daily) versus placebo. At baseline, the median CDAI was 290. The 1.5 mg twice daily arm (data not shown) could not be differentiated from placebo. The 4.5 mg twice daily arm was statistically different from placebo (Table 5), while no additional benefit was seen when the daily budesonide extended-release capsules dose was increased to 15 mg per day (data not shown). Study 3 was a 3-armed parallel group study. The groups were treated with budesonide extended-release capsules 9 mg once daily, budesonide extended-release capsules 4.5 mg twice daily and placebo for 8 weeks, followed by a 2-week double-blind taper phase. The median CDAI at baseline was 263. Neither 9 mg daily nor 4.5 mg twice daily budesonide extended-release capsules dose levels were statistically different from placebo (Table 5). The recommended dosage of budesonide extended-release capsules for the treatment of mild to moderate active Crohn’s disease involving the ileum and/or the ascending colon in adults is 9 mg once daily in the morning for up to 8 weeks [see Dosage and Administration (2.1)].
Two clinical trials (Studies 4 and 5) compared budesonide extended-release capsules with oral prednisolone (initial dose 40 mg per day). Study 4 was a 3-armed parallel group study. The groups were treated with budesonide extended-release capsules 9 mg once daily, budesonide extended-release capsules 4.5 mg twice daily and prednisolone 40 mg (tapered dose) for 8 weeks, followed by a 4-week double blind taper phase. At baseline, the median CDAI was 277. Equal clinical improvement rates (60%) were seen in the budesonide extended-release capsules 9 mg daily and the prednisolone groups in Study 4. In Study 5, 13% fewer patients in the budesonide extended-release capsules group experienced clinical improvement than in the prednisolone group (no statistical difference) (Table 5). The proportion of patients with normal plasma cortisol values (greater than 64.58 ng/mL) was significantly higher in the budesonide extended-release capsules groups in both trials (60% to 66%) than in the prednisolone groups (26% to 28%) at Week 8.
Pediatrics (8 to 17 Years of Age)
The effectiveness of budesonide extended-release capsules, in pediatric patients aged 8 to 17 years, who weigh more than 25 kg with mild to moderate active Crohn’s disease (defined as Crohn's Disease Activity Index (CDAI) ≥ 200) involving the ileum and/or the ascending colon, was assessed in one randomized, double-blind, active control study. This study compared budesonide extended-release capsules 9 mg once daily, with prednisolone, administered at tapering doses starting from 1 mg/kg. Twenty-two (22) patients were treated with budesonide extended-release capsules capsules and 24 patients were treated with prednisolone. After 8 weeks of treatment, 55% (95% CI: 32%, 77%) of patients treated with budesonide extended-release capsules reached the endpoint (CDAI ≤150), as compared to 68% (95% CI: 47%, 89%) of patients treated with prednisolone. The average number of liquid or very soft stools per day (assessed over 7 days) decreased from 1.49 at baseline to 0.96 after treatment with budesonide extended-release capsules and 2.00 at baseline to 0.52 after treatment with prednisolone. The average daily abdominal pain rating (where 0=none, 1=mild, 2=moderate, and 3=severe) decreased from 1.49 at baseline to 0.54 after treatment with budesonide extended-release capsules and 1.64 at baseline to 0.38 after 8 weeks of treatment with prednisolone.
Use of budesonide extended-release capsules in this age group is supported by evidence from adequate and well-controlled studies of budesonide extended-release capsules in adults, and by safety and pharmacokinetic studies performed in pediatric patients.
14.2 Maintenance of Clinical Remission of Mild to Moderate Crohn’s Disease
Adults
The efficacy of budesonide extended-release capsules for maintenance of clinical remission were evaluated in four double-blind, placebo-controlled, 12-month trials in which 380 patients were randomized and treated once daily with 3 mg or 6 mg budesonide extended-release capsules or placebo. Patients ranged in age from 18 to 73 (mean 37) years. Sixty percent of the patients were female and 99% were Caucasian. The mean CDAI at entry was 96. Among the four clinical trials, approximately 75% of the patients enrolled had exclusively ileal disease. Colonoscopy was not performed following treatment. Budesonide extended-release capsules 6 mg per day prolonged the time to relapse, defined as an increase in CDAI of at least 60 units to a total score greater than 150 or withdrawal due to disease deterioration. The median time to relapse in the pooled population of the 4 studies was 154 days for patients taking placebo, and 268 days for patients taking budesonide extended-release capsules 6 mg per day. Budesonide extended-release capsules 6 mg per day reduced the proportion of patients with loss of symptom control relative to placebo in the pooled population for the 4 studies at 3 months (28% versus 45% for placebo).
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Budesonide 3 mg extended-release capsules are hard gelatin capsules with an opaque light grey body and an opaque pink cap, coded with ENTOCORT EC 3 mg on the capsule and are supplied as follows:
- NDC 0574–9855–10 Bottles of 100
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [See USP Controlled Room Temperature].
Keep container tightly closed.
-
17 PATIENT COUNSELING INFORMATION
Advise Patients to read the FDA-Approved patient labeling (Patient Information).
Hypercorticism and Adrenal Axis Suppression
Advise patients that budesonide extended-release capsules may cause hypercorticism and adrenal axis suppression and to follow a taper schedule, as instructed by their healthcare provider if transferring to budesonide extended-release capsules from systemic corticosteroids [see Warnings and Precautions (5.1), (5.2)]. Advise patients that replacement of systemic corticosteroids with budesonide extended-release capsules may unmask allergies (e.g., rhinitis and eczema), which were previously controlled by the systemic drug.
Increased Risk of Infection
Advise patients to avoid exposure to people with chicken pox or measles and, if exposed, to consult their healthcare provider immediately. Inform patients that they are at increased risk of developing a variety of infections; including worsening of existing tuberculosis, fungal, bacterial, viral or parasitic infections or ocular herpes simplex and to contact their healthcare provider if they develop any symptoms of infection [see Warnings and Precautions (5.3)].
Pregnancy
Advise female patients that budesonide extended-release capsules may cause fetal harm and to inform their healthcare provider with a known or suspected pregnancy [see Use in Specific Populations (8.1)].
Administration
- Take budesonide extended-release capsules once daily in the morning.
- Swallow budesonide extended-release capsules whole. Do not chew or crush
- For patients unable to swallow an intact capsule, budesonide extended-release capsules can be opened and administered as follows:
- 1. Place one tablespoonful of applesauce into a clean container (e.g., empty bowl). The applesauce used should not be hot and should be soft enough to be swallowed without chewing.
- 2. Open the capsule(s).
- 3. Carefully empty all the granules inside the capsule(s) on the applesauce.
- 4. Mix the granules with the applesauce.
- 5. Consume the entire contents within 30 minutes of mixing. Do not chew or crush the granules. Do not save the applesauce and granules for future use.
- 6. Follow the applesauce and granules immediately with a glass (8 ounces) of cool water to ensure complete swallowing of the granules.
- Avoid consumption of grapefruit juice for the duration of their budesonide extended-release capsules therapy [see Drug Interactions (7.1)] .
ENTOCORT is a registered trademark of Perrigo Pharma International DAC © Perrigo Pharma International DAC 2016
Revised: 03/2019
-
PATIENT INFORMATION
Budesonide (bue des' oh nide) extended-release capsules
Read this Patient Information before you start taking budesonide extended-release capsules and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What are budesonide extended-release capsules?
Budesonide extended-release capsules are a prescription corticosteroid medicine used to treat mild to moderate Crohn’s disease that affects part of the small intestine (ileum) and part of the large intestine (ascending colon):
- in people 8 years of age and older with active Crohn’s disease
- in adults to help keep symptoms from coming back for up to 3 months
It is not known if budesonide extended-release capsules are safe and effective in children under 8 years of age, or in children 8 to 17 years of age who weigh 55 pounds (25 kg) or less, for the treatment of mild to moderate active Crohn’s disease that affects part of the small intestine (ileum) and part of the large intestine (ascending colon).
It is not known if budesonide extended-release capsules are safe and effective in children to help keep symptoms of mild to moderate Crohn’s disease that affects part of the small intestine (ileum) and part of the large intestine (ascending colon) from coming back.
Who should not take budesonide extended-release capsules?
Do not take budesonide extended-release capsules if:
- you are allergic to budesonide or any of the ingredients in budesonide extended-release capsules. See the end of this leaflet for a complete list of ingredients in budesonide extended-release capsules.
Before you take budesonide extended-release capsules tell your healthcare provider if you have any other medical conditions including if you:
- have liver problems.
- are planning to have surgery.
- have chicken pox or measles or have recently been near anyone with chicken pox or measles.
- have an infection.
- have diabetes or glaucoma or have a family history of diabetes or glaucoma.
- have cataracts.
- have or had tuberculosis.
- have high blood pressure (hypertension).
- have decreased bone mineral density (osteoporosis).
- have stomach ulcers.
- are pregnant or plan to become pregnant. Budesonide extended-release capsules may harm your unborn baby. Talk to your healthcare provider about the possible risk to your unborn baby if you take budesonide extended-release capsules when you are pregnant. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during your treatment with budesonide extended-release capsules.
- are breastfeeding or plan to breastfeed. It is not known if budesonide passes into your breast milk or if it will affect your baby. Talk to your healthcare provider about the best way to feed your baby if you take budesonide extended-release capsules.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Budesonide extended-release capsules and other medicines may affect each other causing side effects.
How should I take budesonide extended-release capsules?
- Take budesonide extended-release capsules exactly as your healthcare provider tells you.
- Your healthcare provider will tell you how many budesonide extended-release capsules capsules to take. Your healthcare provider may change your dose if needed.
- Take budesonide extended-release capsules 1 time each day in the morning.
- Take budesonide extended-release capsules whole. Do not chew or crush budesonide extended-release capsules before swallowing.
- For patients, unable to swallow a whole capsule, budesonide extended-release capsules can be opened and administered as follows:
- 1. Place 1 tablespoonful of applesauce into a clean container, such as an empty bowl. The applesauce used should not be hot and should be soft enough to be swallowed without chewing.
- 2. Open the capsule. You may need to use more than 1 budesonide extended-release capsule for the dose prescribed by your healthcare provider.
- 3. Carefully empty all of the granules inside the capsule on the applesauce.
- 4. Stir the granules with the applesauce.
- 5. Swallow the applesauce and granules mixture within 30 minutes after preparing it. Follow the applesauce and granules immediately with a glass (8 ounces) of cool water to help with complete swallowing of the granules.
- 6. Do not chew or crush the granules.
- 7. Do not save the applesauce and granules for later use.
- If you take too many budesonide extended-release capsules call your healthcare provider right away or go to the nearest hospital emergency room.
What should I avoid while taking budesonide extended-release capsules?
- Do not drink grapefruit juice during your treatment with budesonide extended-release capsules. Drinking grapefruit juice can increase the level of budesonide in your blood.
What are the possible side effects of budesonide extended-release capsules?
Budesonide extended-release capsules may cause serious side effects, including:
- Effects of having too much corticosteroid medicine in your blood (hypercorticism). Long-time use of budesonide extended-release capsules can cause you to have too much corticosteroid medicine in your blood. Tell your healthcare provider if you have any of the following signs and symptoms of hypercorticism:
- o acne
- o thicker or more hair on your body and face
- o bruise easily
- o a fatty pad or hump between your shoulders (buffalo hump)
- o rounding of your face (moon face)
- o pink or purple stretch marks on the skin of your abdomen, thighs, breasts and arms
- o ankle swelling
- Adrenal suppression. When budesonide extended-release capsules are taken for a long period of time (chronic use), adrenal suppression can happen. This is a condition in which the adrenal glands do not make enough steroid hormones. Symptoms of adrenal suppression include: tiredness, weakness, nausea and vomiting and low blood pressure. Tell your healthcare provider if you are under stress or have any symptoms of adrenal suppression during treatment with budesonide extended-release capsules.
- Worsening of allergies. If you take certain other corticosteroid medicines to treat allergies, switching to budesonide extended-release capsules may cause your allergies to come back. These allergies may include a skin condition called eczema or inflammation inside your nose (rhinitis). Tell your healthcare provider if any of your allergies become worse while taking budesonide extended-release capsules.
- Increased risk of infection. Budesonide extended-release capsules weaken your immune system. Taking medicines that weaken your immune system makes you more likely to get infections. Avoid contact with people who have contagious diseases, such as chicken pox or measles, while taking budesonide extended-release capsules. Tell your healthcare provider right away if you come in contact with anyone who has chicken pox or measles.
- Tell your healthcare provider about any signs or symptoms of infection during treatment with budesonide extended-release capsules, including:
- o fever
- o chills
- o pain
- o feeling tired
- o aches
- o nausea and vomiting
The most common side effects of budesonide extended-release capsules in adults include:
- headache
- vomiting
- stomach area (abdominal) pain
- back pain
- infection in your air passages (respiratory infection)
- tiredness
- gas
- indigestion
- nausea
- pain
- dizziness
The most common side effects of budesonide extended-release capsules in children 8 to 17 years of age, who weigh more than 55 pounds (25 kg), are similar to the most common side effects in adults.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of budesonide extended-release capsules. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store budesonide extended-release capsules?
- Store budesonide extended-release capsules at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep budesonide extended-release capsules in a tightly closed container.
Keep budesonide extended-release capsules and all medicines out of the reach of children.
General information about the safe and effective use of budesonide extended-release capsules.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide or Patient Information leaflet. Do not use budesonide extended-release capsules for a condition for which it was not prescribed. Do not give budesonide extended-release capsules to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about budesonide extended-release capsules that is written for health professionals.
What are the ingredients in budesonide extended-release capsules?
Active ingredient: budesonide
Inactive ingredients: ethylcellulose, acetyltributyl citrate, methacrylic acid copolymer type C, triethyl citrate, antifoam M, polysorbate 80, talc, and sugar spheres. The capsule shell contains: gelatin, iron oxide, and titanium dioxide.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured and Distributed by:
Perrigo® Allegan, MI 49010
5V000 RC J4
For more information, go to www.perrigo.com or call 1-866-634-9120.
Revision: 03/2019
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Rx Only
NDC: 0574-9855-10
Budesonide Extended-Release Capsules 3mg
100 Capsules
The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label during the packaging operation.
-
INGREDIENTS AND APPEARANCE
BUDESONIDE
budesonide capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0574-9855 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUDESONIDE (UNII: Q3OKS62Q6X) (BUDESONIDE - UNII:Q3OKS62Q6X) BUDESONIDE 3 mg Inactive Ingredients Ingredient Name Strength ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) ACETYLTRIBUTYL CITRATE (UNII: 0ZBX0N59RZ) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TALC (UNII: 7SEV7J4R1U) GELATIN (UNII: 2G86QN327L) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color GRAY (opaque light gray) Score no score Shape CAPSULE Size 7mm Flavor Imprint Code ENTOCORT;EC;3mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0574-9855-10 1 in 1 CARTON 06/29/2018 1 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021324 06/29/2018 Labeler - Paddock Laboratories, LLC (967694121)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.