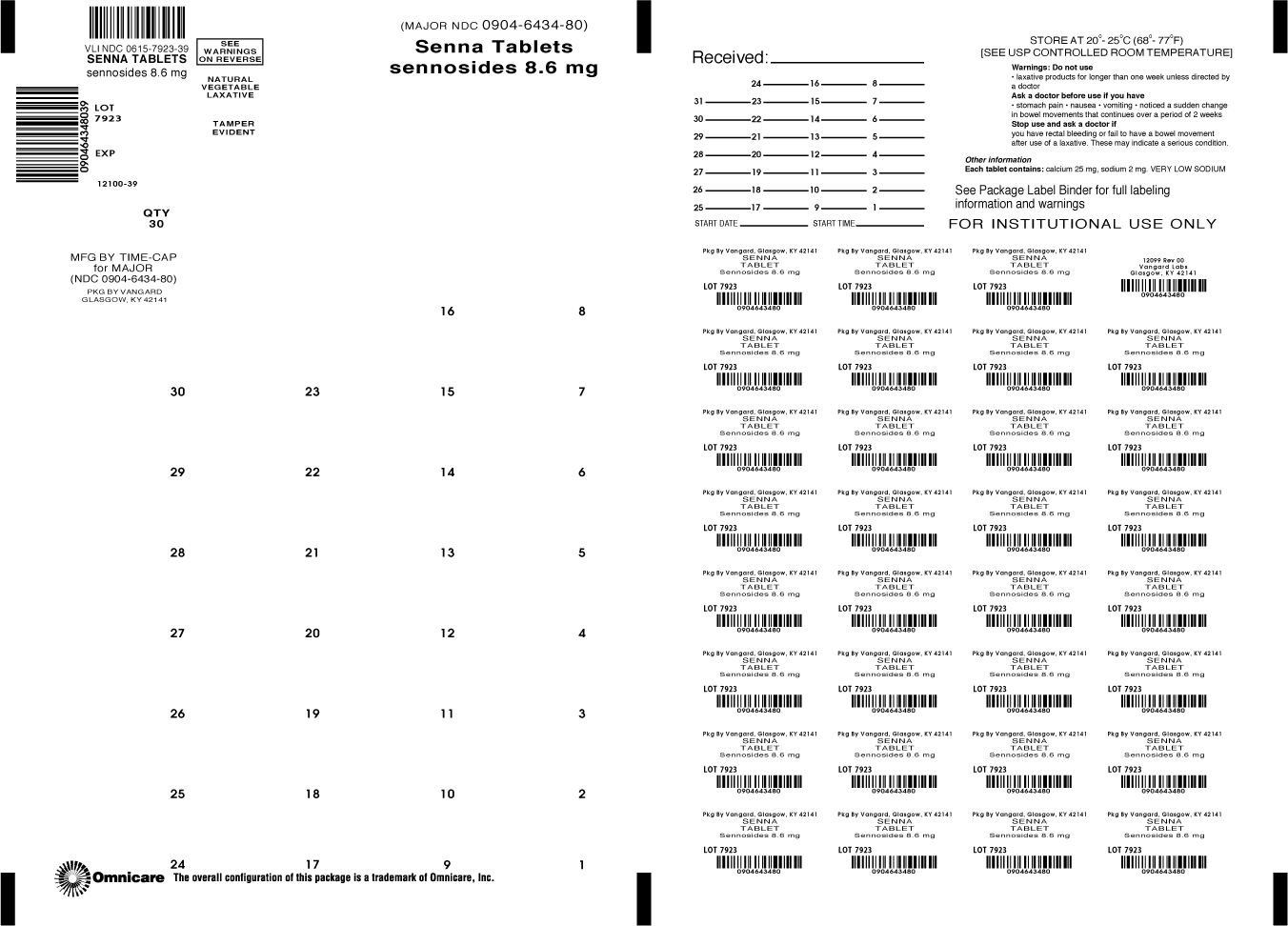
SENNA by NCS HealthCare of KY, Inc dba Vangard Labs SENNA tablet, coated
SENNA by
Drug Labeling and Warnings
SENNA by is a Otc medication manufactured, distributed, or labeled by NCS HealthCare of KY, Inc dba Vangard Labs. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient (in each tablet)
- Purpose
- Uses
- Warnings
-
-
Directions
take preferably at bedtime or as directed by a doctor
If you do not have a comfortable bowel movement by the second day, increase dose by one tablet (do not exceed maximum dosage) or decrease dose until you are comfortable
age starting dosage maximum dosage adults and children over 12 years 2 tablets once a day 4 tablets twice a day children 6 to under 12 years 1 tablet once a day 2 tablets twice a day children 2 to under 6 years 1/2 tablet once a day 1 tablet twice a day children under 2 years ask a doctor ask a doctor - Other information
- Inactive Ingredients
- QUESTIONS
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SENNA
senna tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0615-7923(NDC: 0904-6434) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES A AND B (UNII: 1B5FPI42EN) (SENNOSIDES A AND B - UNII:1B5FPI42EN) SENNOSIDES A AND B 8.6 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MINERAL OIL (UNII: T5L8T28FGP) Product Characteristics Color BROWN Score no score Shape ROUND Size 9mm Flavor Imprint Code TCL080 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0615-7923-39 30 in 1 BLISTER PACK; Type 0: Not a Combination Product 08/31/2015 08/31/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part334 06/09/2015 08/31/2020 Labeler - NCS HealthCare of KY, Inc dba Vangard Labs (050052943) Establishment Name Address ID/FEI Business Operations NCS HealthCare of KY, Inc dba Vangard Labs 050052943 REPACK(0615-7923)
Trademark Results [SENNA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SENNA 97868919 not registered Live/Pending |
Natty Collection LLC 2023-04-02 |
 SENNA 90583748 not registered Live/Pending |
Jeremey Decena 2021-03-17 |
 SENNA 90399285 not registered Live/Pending |
AYRTON SENNA EMPREENDIMENTOS LTDA. 2020-12-21 |
 SENNA 90022160 not registered Live/Pending |
OPWEST DEVELOPMENT LLC 2020-06-26 |
 SENNA 88624114 not registered Live/Pending |
Ceritas Wines LLC 2019-09-19 |
 SENNA 87683504 5564030 Live/Registered |
OMM Imports Inc. 2017-11-14 |
 SENNA 76601884 3268781 Dead/Cancelled |
Studio RTA 2004-07-12 |
 SENNA 75170094 2188775 Live/Registered |
Senna Cosmetics, Inc. 1996-09-23 |
 SENNA 74561186 not registered Dead/Abandoned |
Senna Cosmetics, Inc. 1994-08-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.