BETIMOL- timolol solution/ drops
BETIMOL by
Drug Labeling and Warnings
BETIMOL by is a Prescription medication manufactured, distributed, or labeled by Thea Pharma Inc., Akorn AG. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Betimol ® (timolol ophthalmic solution), 0.25% and 0.5%, is a non-selective beta-adrenergic antagonist for ophthalmic use. The chemical name of the active ingredient is (S)-1-[(1, 1-dimethylethyl)amino]-3-[[4-(4-morpholinyl)-1,2,5-thiadiazol-3-yl]oxy]-2-propanol. Timolol hemihydrate is the levo isomer. Specific rotation is [α] 25 405nm=-16° (C=10% as the hemihydrate form in 1N HCl).
The molecular formula of timolol is Formula C 13H 24N 4O 3S and its structural formula is: 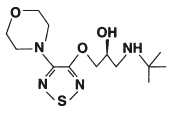
Timolol (as the hemihydrate) is a white, odorless, crystalline powder which is slightly soluble in water and freely soluble in ethanol. Timolol hemihydrate is stable at room temperature.
Betimol ® is a clear, colorless, isotonic, sterile, microbiologically preserved phosphate buffered aqueous solution.
It is supplied in two dosage strengths, 0.25% and 0.5%.
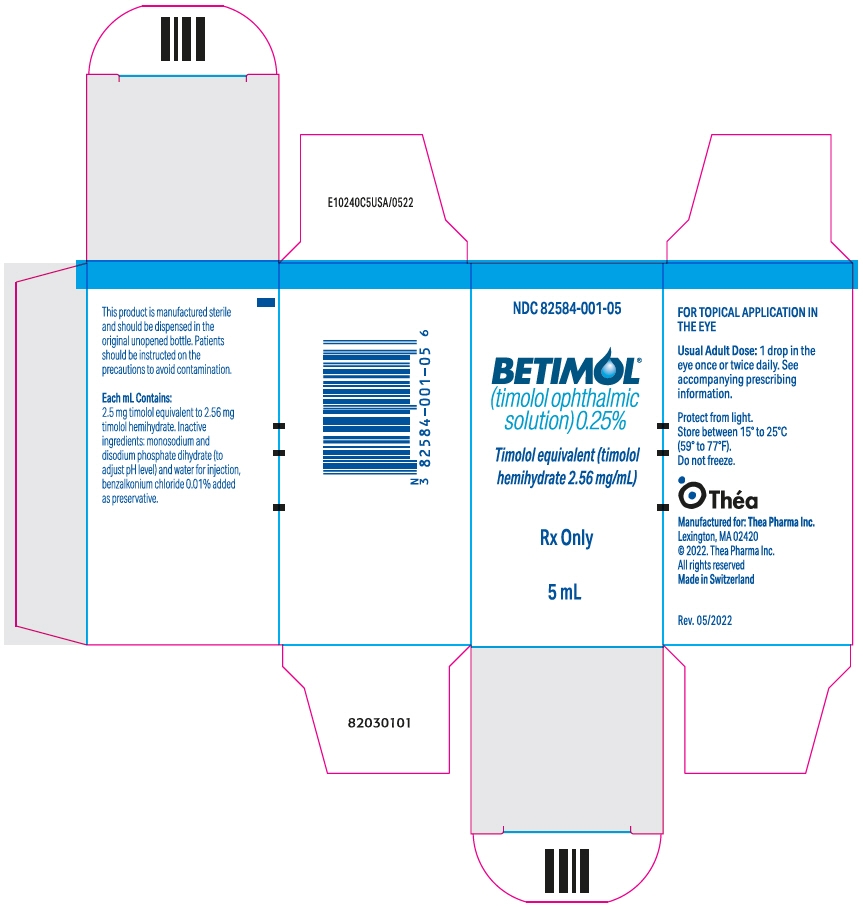
Each mL of Betimol ® 0.25% contains 2.56 mg of timolol hemihydrate equivalent to 2.5 mg timolol.
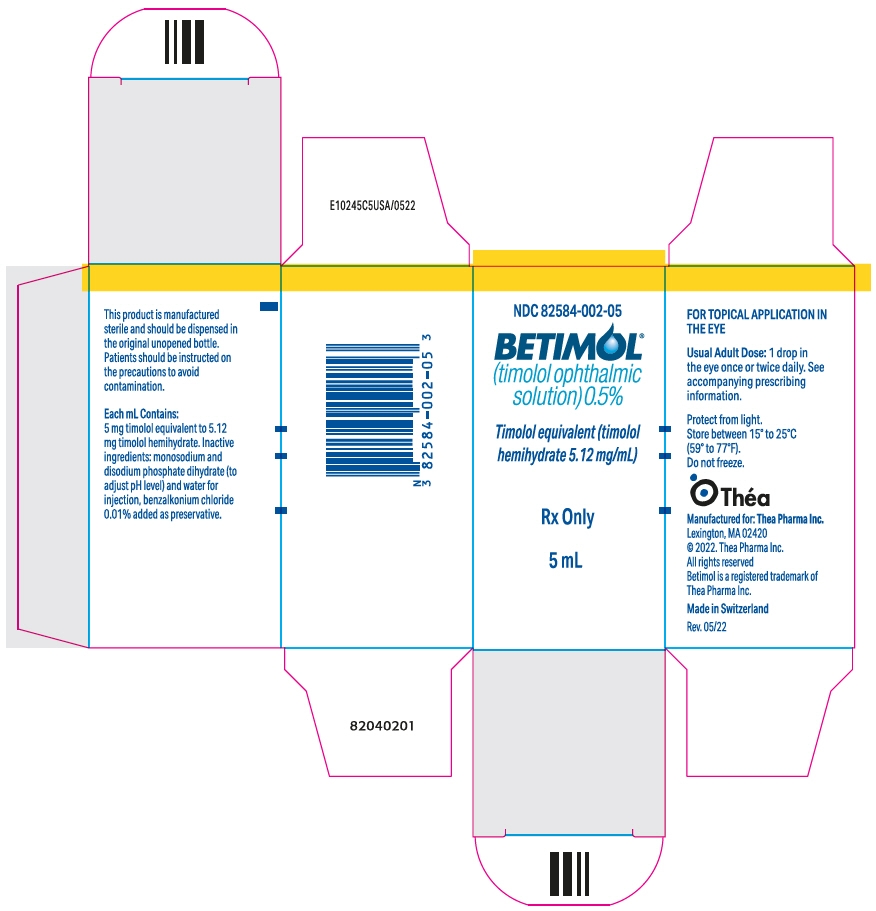
Each mL of Betimol ® 0.5% contains 5.12 mg of timolol hemihydrate equivalent to 5.0 mg timolol.
Inactive ingredients: monosodium and disodium phosphate dihydrate to adjust pH (6.5 - 7.5) and water for injection, benzalkonium chloride 0.01 % added as preservative.
The osmolality of Betimol ® is 260 to 320 mOsmol/kg.
-
CLINICAL PHARMACOLOGY
Timolol is a non-selective beta-adrenergic antagonist.
It blocks both beta 1-and beta 2-adrenergic receptors. Timolol does not have significant intrinsic sympathomimetic activity, local anesthetic (membrane-stabilizing) or direct myocardial depressant activity.
Timolol, when applied topically in the eye, reduces normal and elevated intraocular pressure (IOP) whether or not accompanied by glaucoma. Elevated intraocular pressure is a major risk factor in the pathogenesis of glaucomatous visual field loss. The higher the level of IOP, the greater the likelihood of glaucomatous visual field loss and optic nerve damage. The predominant mechanism of ocular hypotensive action of topical beta-adrenergic blocking agents is likely due to a reduction in aqueous humor production.
In general, beta-adrenergic blocking agents reduce cardiac output both in healthy subjects and patients with heart diseases. In patients with severe impairment of myocardial function, beta-adrenergic receptor blocking agents may inhibit sympathetic stimulatory effect necessary to maintain adequate cardiac function. In the bronchi and bronchioles, beta-adrenergic receptor blockade may also increase airway resistance because of unopposed parasympathetic activity.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Betimol ® is contraindicated in patients with overt heart failure, cardiogenic shock, sinus bradycardia, second- or third-degree atrioventricular block, bronchial asthma or history of bronchial asthma, or severe chronic obstructive pulmonary disease, or hypersensitivity to any component of this product.
-
WARNINGS
As with other topically applied ophthalmic drugs, Betimol ® is absorbed systemically. The same adverse reactions found with systemic administration of beta-adrenergic blocking agents may occur with topical administration. For example, severe respiratory and cardiac reactions, including death due to bronchospasm in patients with asthma, and rarely, death in association with cardiac failure have been reported following systemic or topical administration of beta-adrenergic blocking agents.
Cardiac Failure
Sympathetic stimulation may be essential for support of the circulation in individuals with diminished myocardial contractility, and its inhibition by beta-adrenergic receptor blockade may precipitate more severe cardiac failure.
In patients without a history of cardiac failure, continued depression of the myocardium with beta-blocking agents over a period of time can, in some cases, lead to cardiac failure. Betimol ® should be discontinued at the first sign or symptom of cardiac failure.
Obstructive Pulmonary Disease
Patients with chronic obstructive pulmonary disease (e.g. chronic bronchitis, emphysema) of mild or moderate severity, bronchospastic disease, or a history of bronchospastic disease (other than bronchial asthma or a history of bronchial asthma which are contraindications) should in general not receive beta-blocking agents.
Major Surgery
The necessity or desirability of withdrawal of beta-adrenergic blocking agents prior to a major surgery is controversial. Beta-adrenergic receptor blockade impairs the ability of the heart to respond to beta-adrenergically mediated reflex stimuli. This may augment the risk of general anesthesia in surgical procedures. Some patients receiving beta-adrenergic receptor blocking agents have been subject to protracted severe hypotension during anesthesia. Difficulty in restarting and maintaining the heartbeat has also been reported. For these reasons, in patients undergoing elective surgery, gradual withdrawal of beta-adrenergic receptor blocking agents is recommended. If necessary during surgery, the effects of beta-adrenergic blocking agents may be reversed by sufficient doses of beta-adrenergic agonists.
Diabetes Mellitus
Beta-adrenergic blocking agents should be administered with caution in patients subject to spontaneous hypoglycemia or to diabetic patients (especially those with labile diabetes) who are receiving insulin or oral hypoglycemic agents. Beta-adrenergic receptor blocking agents may mask the signs and symptoms of acute hypoglycemia.
-
PRECAUTIONS
General
Because of the potential effects of beta-adrenergic blocking agents relative to blood pressure and pulse, these agents should be used with caution in patients with cerebrovascular insufficiency. If signs or symptoms suggesting reduced cerebral blood flow develop following initiation of therapy with Betimol ®, alternative therapy should be considered.
There have been reports of bacterial keratitis associated with the use of multiple dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface. (See PRECAUTIONS, Information for Patients.)
Muscle Weakness
Beta-adrenergic blockade has been reported to potentiate muscle weakness consistent with certain myasthenic symptoms (e.g. diplopia, ptosis, and generalized weakness). Beta-adrenergic blocking agents have been reported rarely to increase muscle weakness in some patients with myasthenia gravis or myasthenic symptoms.
In angle-closure glaucoma, the goal of the treatment is to reopen the angle. This requires constricting the pupil. Betimol ® has no effect on the pupil. Therefore, if timolol is used in angle-closure glaucoma, it should always be combined with a miotic and not used alone.
Anaphylaxis
While taking beta-blockers, patients with a history of atopy or a history of severe anaphylactic reactions to a variety of allergens may be more reactive to repeated accidental, diagnostic, or therapeutic challenge with such allergens. Such patients may be unresponsive to the usual doses of epinephrine used to treat anaphylactic reactions.
The preservative benzalkonium chloride may be absorbed by soft contact lenses. Patients who wear soft contact lenses should wait 5 minutes after instilling Betimol ® before they insert their lenses.
Information for Patients
Patients should be instructed to avoid allowing the tip of the dispensing container to contact the eye or surrounding structures.
Patients should also be instructed that ocular solutions can become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions. (See PRECAUTIONS, General.)
Patients requiring concomitant topical ophthalmic medications should be instructed to administer these at least 5 minutes apart.
Patients with bronchial asthma, a history of bronchial asthma, severe chronic obstructive pulmonary disease, sinus bradycardia, second- or third-degree atrioventricular block, or cardiac failure should be advised not to take this product (See CONTRAINDICATIONS.)
Drug Interactions
Beta-adrenergic blocking agents
Patients who are receiving a beta-adrenergic blocking agent orally and Betimol ® should be observed for a potential additive effect either on the intraocular pressure or on the known systemic effects of beta-blockade.
Patients should not usually receive two topical ophthalmic beta-adrenergic blocking agents concurrently.
Catecholamine-depleting drugs
Close observation of the patient is recommended when a beta-blocker is administered to patients receiving catecholamine-depleting drugs such as reserpine, because of possible additive effects and the production of hypotension and/or marked bradycardia, which may produce vertigo, syncope, or postural hypotension.
Calcium antagonists
Caution should be used in the co-administration of beta-adrenergic blocking agents and oral or intravenous calcium antagonists, because of possible atrioventricular conduction disturbances, left ventricular failure, and hypotension. In patients with impaired cardiac function, co-administration should be avoided.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity of timolol (as the maleate) has been studied in mice and rats. In a two-year study orally administrated timolol maleate (300mg/kg/day) (approximately 42,000 times the systemic exposure following the maximum recommended human ophthalmic dose) in male rats caused a significant increase in the incidence of adrenal pheochromocytomas; the lower doses, 25 mg or 100 mg/kg daily did not cause any changes.
In a life span study in mice the overall incidence of neoplasms was significantly increased in female mice at 500 mg/kg/day (approximately 71,000 times the systemic exposure following the maximum recommended human ophthalmic dose). Furthermore, significant increases were observed in the incidences of benign and malignant pulmonary tumors, benign uterine polyps, as well as mammary adenocarcinomas. These changes were not seen at the daily dose level of 5 or 50 mg/kg (approximately 700 or 7,000, respectively, times the systemic exposure following the maximum recommended human ophthalmic dose). For comparison, the maximum recommended human oral dose of timolol maleate is 1 mg/kg/day.
Mutagenic potential of timolol was evaluated in vivo in the micronucleus test and cytogenetic assay and in vitro in the neoplastic cell transformation assay and Ames test. In the bacterial mutagenicity test {Ames test) high concentrations of timolol maleate (5000 and 10,000 g/plate) statistically significantly increased the number of revertants in Salmonella typhimuriumTA100, but not in the other three strains tested. However, no consistent dose-response was observed nor did the number of revertants reach the double of the control value, which is regarded as one of the criteria for a positive result in the Ames test. In vivo genotoxicity tests (the mouse micronucleus test and cytogenetic assay) and in vitro the neoplastic cell transformation assay were negative up to dose levels of 800 mg/kg and 100 g/mL, respectively.
No adverse effects on male and female fertility were reported in rats at timolol oral doses of up to 150 mg/kg/day (21,000 times the systemic exposure following the maximum recommended human ophthalmic dose).
Pregnancy Teratogenic effects
Category C
Teratogenicity of timolol (as the maleate) after oral administration was studied in mice and rabbits. No fetal malformations were reported in mice or rabbits at a daily oral dose of 50 mg/kg (7,000 times the systemic exposure following the maximum recommended human ophthalmic dose). Although delayed fetal ossification was observed at this dose in rats, there were no adverse effects on postnatal development of offspring. Doses of 1000 mg/kg/day (142,000 times the systemic exposure following the maximum recommended human ophthalmic dose) were maternotoxic in mice and resulted in an increased number of fetal resorptions. Increased fetal resorptions were also seen in rabbits at doses of 14,000 times the systemic exposure following the maximum recommended human ophthalmic dose in this case without apparent maternotoxicity.
There are no adequate and well-controlled studies in pregnant women. Betimol ® should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
-
ADVERSE REACTIONS
The most frequently reported ocular event in clinical trials was burning/stinging on instillation and was comparable between Betimol ® and timolol maleate (approximately one in eight patients).
The following adverse events were associated with use of Betimol ® in frequencies of more than 5% in two controlled, double-masked clinical studies in which 184 patients received 0.25% or 0.5% Betimol ®:
OCULAR:
Dry eyes, itching, foreign body sensation, discomfort in the eye, eyelid erythema, conjunctival injection, and headache.
BODY AS A WHOLE:
Headache.
The following side effects were reported in frequencies of 1 to 5%:
OCULAR:
Eye pain, epiphora, photophobia, blurred or abnormal vision, corneal fluorescein staining, keratitis, blepharitis and cataract.
BODY AS A WHOLE:
Allergic reaction, asthenia, common cold and pain in extremities.
CARDIOVASCULAR:
Hypertension.
DIGESTIVE:
Nausea.
METABOLIC/NUTRITIONAL:
Peripheral edema.
NERVOUS SYSTEM/PSYCHIATRY:
Dizziness and dry mouth.
RESPIRATORY:
Respiratory infection and sinusitis.
In addition, the following adverse reactions have been reported with ophthalmic use of beta blockers:
OCULAR:
Conjunctivitis, blepharoptosis, decreased corneal sensitivity, visual disturbances including refractive changes, diplopia and retinal vascular disorder.
BODY AS A WHOLE:
Chest pain.
CARDIOVASCULAR:
Arrhythmia, palpitation, bradycardia, hypotension, syncope, heart block, cerebral vascular accident, cerebral ischemia, cardiac failure and cardiac arrest.
DIGESTIVE:
Diarrhea.
ENDOCRINE:
Masked symptoms of hypoglycemia in insulin dependent diabetics (See WARNINGS).
NERVOUS SYSTEM/PSYCHIATRY:
Depression, impotence, increase in signs and symptoms of myasthenia gravis and paresthesia.
RESPIRATORY:
Dyspnea, bronchospasm, respiratory failure and nasal congestion.
SKIN:
Alopecia, hypersensitivity including localized and generalized rash, urticaria.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Betimol® Ophthalmic Solution is available in concentrations of 0.25% and 0.5%. The usual starting dose is one drop of 0.25% Betimol® in the affected eye(s) twice a day. If the clinical response is not adequate, the dosage may be changed to one drop of 0.5% solution in the affected eye(s) twice a day.
If the intraocular pressure is maintained at satisfactory levels, the dosage schedule may be changed to one drop once a day in the affected eye(s). Because of diurnal variations in intraocular pressure, satisfactory response to the once-a-day dose is best determined by measuring the intraocular pressure at different times during the day.
Since in some patients the pressure-lowering response to Betimol® may require a few weeks to stabilize, evaluation should include a determination of intraocular pressure after approximately 4 weeks of treatment with Betimol®.
Dosages above one drop of 0.5% Betimol® twice a day generally have not been shown to produce further reduction in intraocular pressure. If the patient's intraocular pressure is still not at a satisfactory level on this regimen, concomitant therapy with pilocarpine and other miotics, and/or epinephrine, and/or systemically administered carbonic anhydrase inhibitors, such as acetazolamide, can be instituted.
-
HOW SUPPLIED
Betimol ® (timolol ophthalmic solution) is a clear, colorless solution.
Betimol ® 0.25% is supplied in a white, opaque, plastic, ophthalmic dispenser bottle with a controlled drop tip as follows:
NDC: 82584-001-05 5 mL fill in 5 cc container Betimol ® 0.5% is supplied in a white, opaque, plastic, ophthalmic dispenser bottle with a controlled drop tip as follows:
NDC: 82584-002-05 5 mL fill in 5 cc container NDC: 82584-002-15 15 mL fill in 15 cc container Rx Only
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 5 mL Bottle Carton
NDC: 82584-001-05
BETIMOL ®
(timolol ophthalmic
solution) 0.25%Timolol equivalent (timolol
hemihydrate 2.56 mg/mL)Rx Only
5 mL
-
PRINCIPAL DISPLAY PANEL - 5 mL Bottle Carton - NDC: 82854-002-05
NDC: 82584-002-05
BETIMOL ®
(timolol ophthalmic
solution) 0.5%Timolol equivalent (timolol
hemihydrate 5.12 mg/mL)Rx Only
5 mL
-
INGREDIENTS AND APPEARANCE
BETIMOL
timolol solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 82584-001 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TIMOLOL (UNII: 817W3C6175) (TIMOLOL ANHYDROUS - UNII:5JKY92S7BR) TIMOLOL ANHYDROUS 2.56 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) SODIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: 94255I6E2T) WATER (UNII: 059QF0KO0R) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82584-001-05 1 in 1 CARTON 12/01/2022 06/02/2025 1 5 mL in 1 BOTTLE, DROPPER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020439 12/01/2022 BETIMOL
timolol solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 82584-002 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TIMOLOL (UNII: 817W3C6175) (TIMOLOL ANHYDROUS - UNII:5JKY92S7BR) TIMOLOL ANHYDROUS 5.12 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) SODIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: 94255I6E2T) WATER (UNII: 059QF0KO0R) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82584-002-05 1 in 1 CARTON 11/15/2022 1 5 mL in 1 BOTTLE, DROPPER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 82584-002-15 1 in 1 CARTON 12/01/2022 09/30/2025 2 15 mL in 1 BOTTLE, DROPPER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020439 11/15/2022 Labeler - Thea Pharma Inc. (117787029)
Trademark Results [BETIMOL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BETIMOL 79128616 4442019 Live/Registered |
Akorn International S.Ã .r.l. 2013-02-15 |
 BETIMOL 74628335 1961729 Live/Registered |
AKORN INTERNATIONAL S.A.R.L. 1995-02-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.