REVIVA-SIL- silicone gel pad kit
Reviva-Sil by
Drug Labeling and Warnings
Reviva-Sil by is a Other medication manufactured, distributed, or labeled by Trifluent Pharma LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- USES
- HOW SUPPLIED
-
POSSIBLE REACTIONS INCLUDE
- Superficial maceration of the skin
- Skin discoloration
- Pruritus
- Rash
Rashes may occur on skin under gel-pad due to poor hygiene. Rashes may also result from wrapping gel-pad too tightly around skin. Stop use and ask a doctor if gel-pad is applied properly and skin irritation still occurs.
Discoloration of skin covered by gel-pad may temporarily occur in patients with darker complexions.
Do not use creams, lotions, sun block, or other silicone products on your skin when wearing gel-pad. Only apply gel-pad to clean, bare skin.
-
PRECAUTIONS
Do not apply to open wounds or third degree burns. Never use on a sutured wound until sutures have been removed or when any dermatological conditions disrupt the skin (such as a rash or burn). In rare instances, silicone sheets may cause a rash on the skin. This condition may result from improper cleansing of the scar area where the gel-pad has been applied. Stop use and ask a doctor if product is applied properly and skin irritation still occurs. Persons with dermatological disorders should contact their doctor prior to using this product.
-
DIRECTIONS FOR USE
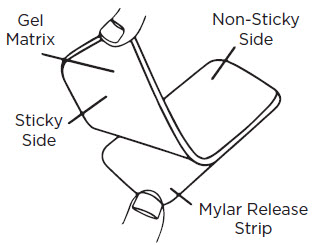
- 1) Wash your hands and the scar area with soap and water. Dry thoroughly.
- 2) Cut the RevivaSil™ Gel-Pad to fit the scar area as needed, so that the edges extend at least 1/4" beyond the scar on all sides.
- 3) Gently peel the gel-pad from the Mylar release strip (it is normal for small bits of the gel to remain on the Mylar release strip). Do not discard the Mylar release strip.
- 4) Place the sticky side of the gel-pad directly onto the scar. For best results, wear the gel-pad 8-12 hours per treatment (12 hours is recommended). Wait 8-12 hours without use before next treatment.
- 5) After removal of the gel-pad, apply 1-2 drops of Reviva-E™ onto the scar; massage gently until absorbed. Repeat as needed.
- 6) When not wearing the gel-pad, place the gel-pad (sticky side) back onto the Mylar release strip. Place the gel-pad back into the original pouch for protection.
- 7) When the sticky side of the gel-pad appears dirty or worn, use your finger to gently wash the gel-pad with soap and water, as needed. Rinse with water and allow to air dry. The stickiness will return once dry. Do not fold while washing or drying.
* Proper use, and gentle care of the gel-pad will be re-usable up to eight (8) days.
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
- COMPONENTS
-
DIRECTIONS
- 1) Wash your hands and the scar area with soap and water. Dry thoroughly.
- 2) Cut the RevivaSil™ Gel-Pad to fit the scar area as needed, so that the edges extend at least 1/4" beyond the scar on all sides.
- 3) Gently peel the gel-pad from the Mylar release strip (it is normal for small bits of the gel to remain on the Mylar release strip). Do not discard the Mylar release strip.
- 4) Place the sticky side of the gel-pad directly onto the scar. For best results, wear the gel-pad 8-12 hours per treatment (12 hours is recommended). Wait 8-12 hours without use before next treatment.
- 5) After removal of the gel-pad, apply 1-2 drops of Reviva-E™ onto the scar; massage gently until absorbed. Repeat as needed.
- 6) When not wearing the gel-pad, place the gel-pad (sticky side) back onto the Mylar release strip. Place the gel-pad back into the original pouch for protection.
- 7) When the sticky side of the gel-pad appears dirty or worn, use your finger to gently wash the gel-pad with soap and water, as needed. Rinse with water and allow to air dry. The stickiness will return once dry. Do not fold while washing or drying.
-
PRECAUTIONS
Do not apply to open wounds or third degree burns. Never use on a sutured wound until sutures have been removed or when any dermatological conditions disrupt the skin (such as a rash or burn). In rare instances, silicone sheets may cause a rash on the skin. This condition may result from improper cleansing of the scar area where the gel-pad has been applied. Stop use and ask a doctor if product is applied properly and skin irritation still occurs. Persons with dermatological disorders should contact their doctor prior to using this product.
-
PRINCIPAL DISPLAY PANEL - Kit Label
TRIFLUENT
PHARMA®RevivaSil™
GEL-PAD KITQUESTIONS OR COMMENTS:
CALL (210) 944-6920MANUFACTURED FOR:
Trifluent Pharma, LLC
San Antonio, TX 78213MADE IN THE U.S.A.
540-01 REV02 12-22-2023CONTAINS:
FOUR (4) SILICONE GEL-PADS (3" X 5.5") (NON-STERILE)
ONE (1) REVIVA-E™ SOLUTION – 0.5 FL. OZ. (15 mL)PRODUCT SHOULD BE ADMINISTERED UNDER THE
SUPERVISION OF A LICENSED MEDICAL PRACTITIONER
(CONTENTS NOT FOR INDIVIDUAL SALE OR DISTRIBUTION)NDC: 73352-540-01

- PRINCIPAL DISPLAY PANEL - 15 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
REVIVA-SIL
elastomer, silicone, for scar management kitProduct Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:73352-540 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:73352-540-01 1 in 1 BOX Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 4 POUCH 4 Part 2 1 BOTTLE, DROPPER 15 mL Part 1 of 2 REVIVASIL
elastomer, silicone, for scar management patchProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 12 h Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 POUCH; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date EXEMPT DEVICE MDA 12/25/2023 Part 2 of 2 REVIVA-E
elastomer, silicone, for scar management solution/ dropsProduct Information Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength DIMETHICONE 100 (UNII: RO266O364U) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 4 (UNII: CZ227117JE) DIMETHICONOL (40 CST) (UNII: 343C7U75XW) TOCOPHEROL (UNII: R0ZB2556P8) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date EXEMPT DEVICE MDA 12/25/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date EXEMPT DEVICE MDA 12/25/2023 Labeler - Trifluent Pharma LLC (117167281)
Trademark Results [Reviva-Sil]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 REVIVA-SIL 98215293 not registered Live/Pending |
Real Value Products Corporation 2023-10-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
