ACETYLCYSTEINE injection, solution
Acetylcysteine by
Drug Labeling and Warnings
Acetylcysteine by is a Prescription medication manufactured, distributed, or labeled by STELIS BIOPHARMA LIMITED, Steriscience Pte. Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ACETYLCYSTEINE INJECTION safely and effectively. See full prescribing information for Acetylcysteine Injection.
ACETYLCYSTEINE INJECTION for intravenous use
Initial U.S. Approval: 2004RECENT MAJOR CHANGES
Dosage and Administration (2.1, 2.2, 2.3, 2.4, 2.5) 12/2024
INDICATIONS AND USAGE
Acetylcysteine injection is an antidote for acetaminophen overdose indicated to prevent or lessen hepatic injury after ingestion of a potentially hepatotoxic quantity of acetaminophen in adults and pediatric patients who weigh 5 kg or greater with an acute ingestion or from repeated supratherapeutic ingestion (RSI) (1). (1)
DOSAGE AND ADMINISTRATION
Pre-Treatment Assessment Following Acute Ingestion (2.1):
Prior to initiating treatment with Acetylcysteine injection, decide whether the three-bag or two-bag regimen will be used.
Obtain a plasma or serum sample to assay for acetaminophen concentration at least 4 hours after ingestion.
If the time of acetaminophen ingestion is unknown:
o Administer a loading dose of Acetylcysteine injection immediately.
o Obtain an acetaminophen concentration to determine need for continued treatment.
If the acetaminophen concentration cannot be obtained (or is unavailable or uninterpretable) within the 8-hour time interval after acetaminophen ingestion or there is clinical evidence of acetaminophen toxicity:
o Administer a loading dose of Acetylcysteine injection immediately and continue treatment for a total of two doses over 20 hours or three doses over 21 hours (2.5).
If the patient presents more than 8 hours after ingestion and the time of acute acetaminophen ingestion is known:
o Administer a loading dose of Acetylcysteine injection immediately
o Obtain acetaminophen concentration to determine need for continued treatment
If the patient presents less than 8 hours after ingestion and the time of acute acetaminophen ingestion is known and the acetaminophen concentration is known:
o Use the revised Rumack-Matthew nomogram (Figure 1) to determine whether or not to initiate treatment with Acetylcysteine injection (2.2) (2)Nomogram for Estimating Potential for Hepatotoxicity from Acute Acetaminophen Ingestion (2.2):
See Full Prescribing Information for instructions on how to use the nomogram to determine the need for dosing. (2)Preparation and Storage of Diluted Solution Prior to Administration (2.3):
Calculate the dose (mg) based on the patient’s weight in kg; multiple vials of Acetylcysteine injection may be required.
o Acetylcysteine injection is hyperosmolar (2,600 mOsmol/L), therefore Acetylcysteine injection must be diluted in the recommended volume of sterile water for injection, 0.45% sodium chloride injection, or 5% dextrose in water injection prior to intravenous administration. In general, 0.45% normal saline is the preferred diluent because it provides a more consistent osmolarity profile, reduces the amount of free water delivered to the patient, and better approximates physiologic fluids. (2)See Full Prescribing Information for examples of osmolarity depending on the type of solution and Acetylcysteine injection concentration. (2)
General Considerations for Selecting the Three-Bag or Two-Bag Regimen (2.4):
It is not known whether the two-bag regimen is comparable to the three-bag regimen in preventing hepatotoxicity.
Patients 40 kg or less should receive the three-bag regimen.
For patients weighing 41 kg or greater, the three-bag regimen may be preferred for those with early signs of severe liver injury or a large acetaminophen ingestion.
Recommended Dosage for Acute Acetaminophen ingestion (2.5):
Acetylcysteine injection is for intravenous administration only.
Total dosage of Acetylcysteine injection is 300 mg/kg given intravenously as either:
o 3 separate doses infused over a total of 21 hours
OR
o 2 separate doses infused over a total of 20 hours.
See Full Prescribing Information for weight-based dosage and weight-based dilution (2.5)
See Full Prescribing Information for recommendations for continuing Acetylcysteine injection treatment after 21 hours (2.2)
Repeated Supratherapeutic Acetaminophen Ingestion (2.6):
Obtain acetaminophen concentration and other laboratory tests to guide treatment; revised Rumack-Matthew nomogram does not apply. (2)DOSAGE FORMS AND STRENGTHS
Injection: 6000 mg/30 mL (200 mg/mL) in a single-dose vial (3) (3)
CONTRAINDICATIONS
Patients with a previous hypersensitivity reaction to acetylcysteine (4) (4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity Reactions: Fatal or life-threatening anaphylaxis, rash, hypotension, wheezing, shortness of breath and/or bronchospasm have been observed. Observe patients during and after the infusion; immediately discontinue infusion if a serious reaction occurs and initiate appropriate treatment. Acetylcysteine injection infusion may be carefully restarted after treatment of hypersensitivity has been initiated and acute symptoms have resolved (5.1).
- Fluid Overload:Total volume administered should be reduced for patients weighing less than 40 kg and for those requiring fluid restriction (5.2).
ADVERSE REACTIONS
Revised: 9/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Pre-Treatment Assessment and Testing Following Acute Acetaminophen Ingestion
2.2 Nomogram for Estimating Potential for Hepatoxicity from Acute Acetaminophen Ingestion and Need for Acetylcysteine injection Treatment
2.3 Preparation and Storage of Acetylcysteine injection Diluted Solution Prior to Administration
2.4 General Considerations for Selecting the Three-Bag or Two-Bag Regimen
2.5 Recommended Dosage For Acute Acetaminophen Ingestion
2.6 Recommendations for Repeated Supratherapeutic Acetaminophen Ingestion
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Fluid Overload
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Pre-Treatment Assessment and Testing Following Acute Acetaminophen Ingestion
Prior to initiating treatment with Acetylcysteine injection, decide whether the three-bag or two-bag regimen will be used [see Dosage and Administration (2.4)].
The following recommendations are related to acute acetaminophen ingestion. For recommendations related to repeated supratherapeutic exposure [see Dosage and Administration (2.6)].
1. Assess the history and timing of acetaminophen ingestion as an overdose.
The reported history of the quantity of acetaminophen ingested as an overdose is often inaccurate and is not a reliable guide to therapy.
2. Obtain the following laboratory tests to monitor hepatic and renal function and electrolyte and fluid balance: aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, international normalized ratio (INR), creatinine, blood urea nitrogen (BUN), blood glucose, and electrolytes.
3. Obtain a plasma or serum sample to assay for acetaminophen concentration at least 4 hours after ingestion. Acetaminophen concentrations obtained earlier than 4 hours post-ingestion may be misleading as they may not represent maximum acetaminophen concentrations.
4. If the time of acute acetaminophen ingestion is unknown:
Administer a loading dose of Acetylcysteine injection immediately [see Dosage and Administration (2.5)].
Obtain an acetaminophen concentration to determine need for continued treatment [see Dosage and Administration (2.2)].5. If the acetaminophen concentration cannot be obtained (or is unavailable or uninterpretable) within the 8- hour time interval after acetaminophen ingestion or there is clinical evidence of acetaminophen toxicity:
Administer a loading dose of Acetylcysteine injection immediately and continue treatment for a total of two doses over 20 hours or three doses over 21 hours [see Dosage and Administration (2.5)].6. If the patient presents more than 8 hours after ingestion and the time of acute acetaminophen ingestion is known:
Administer a loading dose of Acetylcysteine injection immediately [see Dosage and Administration (2.5)]
Obtain an acetaminophen concentration to determine need for continued treatment [see Dosage and Administration (2.2)].7. If the patient presents less than 8 hours after ingestion and the time of acute acetaminophen ingestion is known and the acetaminophen concentration is known:
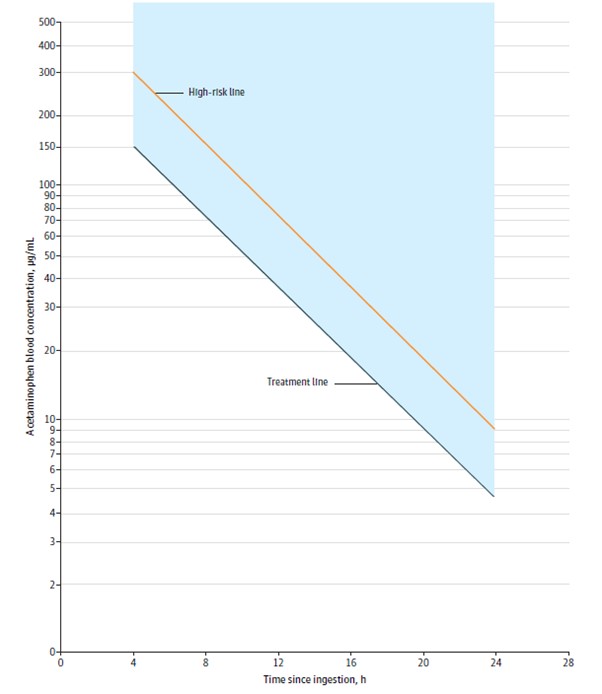
Use the revised Rumack-Matthew nomogram (Figure 1) to determine whether or not to initiate treatment with Acetylcysteine injection [see Dosage and Administration (2.2)].2.2 Nomogram for Estimating Potential for Hepatoxicity from Acute Acetaminophen Ingestion and Need for Acetylcysteine injection Treatment
Acetylcysteine injection is an antidote for acetaminophen overdose. The critical ingestion-treatment interval for maximal protection against severe hepatic injury is between 0 and 8 hours. Efficacy diminishes progressively after 8 hours and treatment initiation between 15 and 24 hours post-ingestion of acetaminophen yields limited efficacy. However, it does not appear to worsen the condition of patients and it should not be withheld, since the reported time of ingestion may not be correct.
If the timing of the acute acetaminophen ingestion is known and the results of the acetaminophen assay are available within 8 hours:
Refer to the revised Rumack-Matthew nomogram (see Figure 1) to determine whether or not to initiate treatment with Acetylcysteine injection.
Initiation of Acetylcysteine injection depends on the plasma or serum acetaminophen concentration and also the clinical presentation of the patient.
The nomogram may underestimate the hepatotoxicity risk in patients with chronic alcoholism, malnutrition, or CYP2E1 enzyme inducing drugs (e.g., isoniazid), and consideration should be given to treating these patients even if the acetaminophen concentrations are in the nontoxic range.Loading dose
For patients whose acetaminophen concentrations are at or above the treatment line (see Figure 1):
Administer a loading dose of Acetylcysteine injection [see Dosage and Administration (2.5)].
For patients with an acute overdose from an extended-release acetaminophen, if the acetaminophen concentration at 4 hours post ingestion is below the treatment line (see Figure 1) then obtain a second sample for acetaminophen concentration 8 to 10 hours after the acute ingestion. If the second value is at or above the treatment line (see Figure 1):
Administer a loading dose of Acetylcysteine injection [see Dosage and Administration (2.5)].
For patients whose values are below the treatment line (see Figure 1), but time of ingestion was unknown or sample was obtained less than 4 hours after ingestion:
Administer a loading dose of Acetylcysteine injection [see Dosage and Administration (2.5)].
For patients whose values are below thetreatment line (see Figure 1) and time of ingestion is known and the sample was obtained more than 4 hours after ingestion, do not administer Acetylcysteine injection because there is minimal risk of hepatotoxicity.Figure 1. Revised Rumack-Matthew Nomogram for Estimating Risk of Hepatoxicity After Acute Ingestion of Acetaminophen
Maintenance Dose
Determine the need for continued treatment with Acetylcysteine injection after the loading dose. Choose ONE of the following based on the acetaminophen concentration:
The acetaminophen concentration is above the treatment line (see Figure 1) according to the nomogram
- Continue Acetylcysteine injection treatment with the maintenance dose for a total of either two or three separate doses over an infusion time of 20 or 21 hours, respectively [see Dosage and Administration (2.5)].
- Monitor hepatic and renal function and electrolytes throughout treatment.
The acetaminophen concentration could not be obtained:
- Continue Acetylcysteine injection treatment with the maintenance dose for a total of either two or three separate doses over an infusion time of 20 or 21 hours [see Dosage and Administration (2.4)].
- Monitor hepatic and renal function and electrolytes throughout treatment.
For patients whose acetaminophen concentration is below the treatment line (see Figure 1) (see Figure 1) and time of ingestion is known and the sample was obtained more than 4 hours after ingestion:
- Discontinue Acetylcysteine injection.
The acetaminophen concentration was in the non-toxic range, but time of ingestion was unknown or less than 4 hours:
- Obtain a second sample for acetaminophen concentration and consider the patient’s clinical status to decide whether or not to continue Acetylcysteine injection treatment.
- If there is any uncertainty as to patient’s risk of developing hepatotoxicity, it is recommended to administer a complete treatment course.
Continued Therapy After Completion of Loading and Maintenance Doses
In cases of suspected massive overdose, or with concomitant ingestion of other substances, or in patients with preexisting liver disease; the absorption and/or the half-life of acetaminophen may be prolonged. In such cases, consideration should be given to the need for continued treatment with Acetylcysteine injection beyond the total 300 mg/kg dose.
Acetaminophen levels and ALT/AST and INR should be checked after the last maintenance dose. If acetaminophen levels are still detectable, or if the ALT/AST are still increasing or the INR remains elevated; dosing should be continued and the treating physician should contact a US regional poison center at 1-800-222-1222, alternatively, a “special health professional assistance line for acetaminophen overdose” at 1-800-525-6115 for assistance with dosing recommendations, or 1-877-484-2700 for additional information.
2.3 Preparation and Storage of Acetylcysteine injection Diluted Solution Prior to Administration
Refer to Table 2 and Table 3 to calculate the dose (mg) based on the patients weight in kg; multiple vials of Acetylcysteine injection may be required [see Dosage and Administration ( 2.5)] .Discard any unused portion left in the vial.
Because Acetylcysteine injection is hyperosmolar (2,600 mOsmol/L), Acetylcysteine injection must be diluted in the recommended volume of sterile water for injection, 0.45% sodium chloride injection (1/2 normal saline), or 5% dextrose in water prior to intravenous administration. The total injection volume will vary based on the patients weight and chosen dosage regimen (i.e., three-bag or two-bag) [see Dosage and Administration (2.5), Warnings and Precautions (5.2)].
The choice of diluent should be based on the individual patients clinical status, concurrent medical conditions, and institutional protocols. The treating clinician should assess each case individually and consult with their pharmacy if there are any concerns about the appropriate diluent choice. In general, 0.45% normal saline is the preferred diluent because it provides a more consistent osmolarity profile, reduces the amount of free water delivered to the patient, and better approximates physiologic fluids than 5% dextrose in water or sterile water for injection. However, consider 5% dextrose in water or sterile water for injection if sodium load is a concern for the patient.
Dilution of Acetylcysteine injection in each of these three solution results in different osmolarity of the acetylcysteine solution for intravenous administration (see Table 1 for examples of different osmolarity of the solution depending on the type of solution and the Acetylcysteine injection concentration).Table 1. Examples of Acetylcysteine Concentration and Osmolarity in Three Solutions Acetylcysteine Concentration Osmolality Sterile Water for Injection 0.45% Sodium Chloride Injection 5% Dextrose in Water (D5W) 4 mg/mL (lowest concentration 3-bag protocol) 52 mOsmol/L* 194 mOsmol/L 311 mOsmol/L 54.5 mg/mL (highest concentration 3-bag protocol) 744 mOsmol/L 855 mOsmol/L 957 mOsmol/L 7.9 mg/mL (lowest concentration 2-bag protocol) 105 mOsmol/L 241 mOsmol/L 360 mOsmol/L 18.2 mg/mL (highest concentration 2-bag protocol) 239 mOsmol/L 368 mOsmol/L 487 mOsmol/L *Adjust osmolarity to a physiologically safe level (generally not less than 150 mOsmol/L in pediatric patients).
The choice of diluent should be based on the individual patient’s clinical status, concurrent medical conditions, and institutional protocols. The treating clinician should assess each case individually and consult with their pharmacy if there are any concerns about the appropriate diluent choice. In general, 0.45% normal saline is the preferred diluent, as it provides a more consistent osmolarity profile, reduces the amount of free water delivered to the patient, and better approximates physiologic fluids than 5% dextrose in water or sterile water for injection.
Visually inspect for particular matter and discoloration prior to administration. The color of the diluted solution range from colorless to a slight pink or purple once the stopper is punctured (the color change does not affect the quality of the product). The diluted solution can be stored for 24 hours at room temperature. Discard the unused portion. If a vial was previously opened, do not use for intravenous administration.
2.4 General Considerations for Selecting the Three-Bag or Two-Bag Regimen
The total recommended dosage of Acetylcysteine injection is 300 mg/kg given intravenously as either a three-bag regimen or a two-bag regimen [see Dosage and Administration ( 2.5)] .
It is not known whether the two-bag regimen is comparable to the three-bag regimen for efficacy in preventing hepatotoxicity.
There are insufficient data to recommend use of the two-bag regimen in patients 40 kg or less; these patients should receive the three-bag regimen.
For patients weighing 41 kg or greater, the clinician should consider the following factors when deciding whether to select the three-bag or two-bag regimen:- The three-bag regimen administers more Acetylcysteine injection in the first three hours and may be preferred for patients with early signs of severe liver injury or history of a large acetaminophen ingestion; however, there is the potential for a higher incidence of hypersensitivity reactions.
- The two-bag regimen delivers a lower amount of Acetylcysteine injection over the first three hours and may be associated with fewer hypersensitivity reactions than the three-bag regimen [see Adverse Reactions ( 6.2), Clinical Studies ( 14)] .
2.5 Recommended Dosage For Acute Acetaminophen Ingestion
Acetylcysteine injection is for intravenous administration only.
The total recommended dosage of Acetylcysteine injection is 300 mg/kg given intravenously as either a three-bag regimen administered as a loading, second, and third dose infused over a total of 21 hours or a two-bag regimen administered as a loading and second dose infused over a total of 20 hours.
For the recommended weight-based dosage and weight-based dilution in patients see Table 2 for the three-bag regimen (for pa.tients 5 kg or greater) or Table 3 for the two-bag regimen (for patients 41 kg or greater).
Three Bag Regimen
Table 2. Three-Bag Recommended Acetylcysteine injection Dosage and Dilution for Patients 5 kg or greater
Body WeightBag 1 (Loading Dose) Bag 2 (Second Dose) Bag 3 (Third Dose) Loading Dose Diluent Volume Infusion Time Second Dose Diluent Volume* Infusion time Third Dose Diluent Volume* Infusion time 5 kg**to 20 kg 150 mg/kg 3 mL/kg Infused Over 1 hour 250 mg 7 mL/kg Infused
Over 4 hours500 mg 14 mL/kg Infused
Over 16 hours21 kg to 40 kg 150 mg/kg 100 mL 500 mg 250 mL 1,000mg 500 mL 41 kg to 99 kg 150 mg/kg 200 mL 750 mg 500 mL 1,500 mg 1,000 mL 100 kg or greater*** 15,000 mg 200 mL 1,000mg 500 mL 2,000 mg 1,000 mL * Dilute Acetylcysteine injection in one of the following three solutions: sterile water for injection, 0.45% sodium chloride injection, or 5% dextrose in water.
** Recommended dosing for those less than 5 kg has not been studied.
*** No specific studies have been conducted to evaluate the necessity of dose adjustments in patients weighing over 100 kg. Limited information is available regarding the dosing requirements of patients that weigh more than 100 kg.
Two-Bag Regimen
Table 3. Alternative Regimen for Patients 41 kg or Greater: Two-Bag Recommended ACETYLCYSTEINE INJECTION Dosage and Dilution Body Weight Bag 1 (Loading Dose) Bag 2 (Second Dose) Loading Dose Diluent Volume * Infusion Time Second Dose Diluent Volume * Infusion Time 41 kg to 99 kg 200 mg/kg 1,000 mL Infused Over 4 hours 100 mg/kg 500 mL Infused Over 16 hours 100 kg or greater** 20,000 mg 1,000 mL 10,000 mg 500 mL * Dilute Acetylcysteine injection in one of the following three solutions: sterile water for injection, 0.45% sodium chloride injection, or 5% dextrose in water.
**No specific studies have been conducted to evaluate the necessity of dose adjustments in patients weighing over 100 kg. Limited information is available regarding the dosing requirements of patients that weigh more than 100 kg2.6 Recommendations for Repeated Supratherapeutic Acetaminophen Ingestion
Repeated supratherapeutic acetaminophen ingestion (RSI) is an ingestion of acetaminophen at dosages higher than those recommended for extended periods of time. The risk of hepatotoxicity and the recommendations for treatment of acute acetaminophen ingestion (i.e., the revised Rumack-Matthew nomogram) do not apply to patients with RSI.
Therefore, obtain the following information to guide Acetylcysteine injection treatment for RSI:
- Acetaminophen serum or plasma concentrations. A reported history of the quantity of acetaminophen ingested is often inaccurate and is not a reliable guide to therapy.
- Laboratory tests to monitor hepatic and renal function and electrolyte and fluid balance: AST, ALT, bilirubin, INR, creatinine, BUN, blood glucose, and electrolytes.
For specific Acetylcysteine injection dosage and administration information in patients with RSI, consider contacting your regional poison center at 1-800-222-1222, or alternatively, a special health professional assistance line for acetaminophen overdose at 1-800-525-6115.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Serious acute hypersensitivity reactions;including fatal or life-threatening anaphylaxis,rash,hypotension,wheezing,and/or shortness of breath;have been observed in patients receiving intravenous acetylcysteine for acetaminophen overdose and occurred soon after initiation of the infusion [see Adverse Reactions (6)]. If a severe hypersensitivity reaction occurs, immediately stop the infusion of Acetylcysteine injection and initiate appropriate treatment.
Patients with asthma should be closely monitored during initiation of Acetylcysteine injection therapy and throughout Acetylcysteine injection therapy.Acute flushing and erythema of the skin may occur in patients receiving acetylcysteine intravenously. These reactions usually occur 30 to 60 minutes after initiating the infusion and often resolve spontaneously despite continued infusion of acetylcysteine. If a reaction to acetylcysteine involves more than simply flushing and erythema of the skin, it should be treated as a hypersensitivity reaction.
Management of less severe hypersensitivity reactions should be based upon the severity of the reaction and include temporary interruption of the infusion and/or administration of antihistaminic drugs. The Acetylcysteine injection infusion may be carefully restarted after treatment of the hypersensitivity symptoms has been initiated and acute symptoms have resolved; however, if the hypersensitivity reaction returns upon re-initiation of treatment or increases in severity, Acetylcysteine injection should be discontinued and alternative patient management should be considered.
5.2 Fluid Overload
The total volume of Acetylcysteine injection administered should be adjusted for patients less than 40 kg and for those requiring fluid restriction.
Intravenous administration of Acetylcysteine injection can cause fluid overload, potentially resulting in hyponatremia, seizure and death. To avoid fluid overload, use the recommended dilution [see Dosage and Administration (2.5)].
-
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
The following clinically significant adverse reactions are described elsewhere in labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Fluid Overload [see Warnings and Precautions (5.2)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In the literature the most frequently reported adverse reactions attributed to intravenous acetylcysteine administration were rash, urticaria and pruritus. The frequency of adverse reactions has been reported to be between 0.2% and 21%, and they most commonly occur during the initial loading dose of acetylcysteine.
Loading Dose/Infusion Rate Study
In a randomized, open-label, multi-center clinical study conducted in Australia in patients with acetaminophen poisoning, the rates of hypersensitivity reactions between a 15-minute and 60-minute intravenous infusion for the 150 mg/kg loading dose of acetylcysteine were compared.
The incidence of drug-related adverse reactions occurring within the first 2 hours following acetylcysteine administration is presented in Table 4. Overall, 17% of patients developed an acute hypersensitivity reaction (18% in the 15-minute infusion group; 14% in the 60-minute infusion group) [see Warnings and Precautions (5.1), Clinical Studies (14)].
Table 4. Incidence of Drug-Related Adverse Reactions Occurring Within the First 2 Hours Following Study Drug Administration by Preferred Term: Loading Dose/Infusion Rate Study Treatment Group 15-minutes 60-minutes Number of Patients n=109 n=71 Cardiac disorders 5 (5%) 2 (3%) Severity: Tachycardia NOS Unkn Mild Moderate Severe Unkn Mild Moderate Severe 4 (4%) 1 (1%) 2 (3%) Gastrointestinal disorders 16 (15%) 7 (10%) Severity: Nausea Vomiting NOS Unkn Mild Moderate Severe Unkn Mild Moderate Severe 1 (1%) 6 (6%) 1 (1%) 1 (1%) 2 (2%) 11 (10%) 2 (3%) 4 (6%) Immune System Disorders 20 (18%) 10 (14%) Severity: Hypersensitivity reaction Unkn Mild Moderate Severe 2 (2%) 6 (6%) 11 (10%) 1 (1%) 4 (6%) 5 (7%) 1 (1%) Respiratory, thoracic and mediastinal disorders 2 (2%) 2 (3%) Severity: Unkn Mild Moderate Severe Unkn Mild Moderate Severe Pharyngitis Rhinorrhea Rhonchi Throat tightness 1 (1%) 1 (1%) 1 (1%) 1 (1%) Skin & subcutaneous tissue disorders 6 (6%) 5 (7%) Severity: Pruritus Rash NOS Unkn Mild Moderate Severe Unkn Mild Moderate Severe 1 (1%) 2 (3%) 3 (3%) 2 (2%) 3 (4%) Vascular disorders 2 (2%) 3 (4%) Severity: Flushing Unkn Mild Moderate Severe Unkn Mild Moderate Severe 1 (1%) 1 (1%) 2 (3%) 1 (1%) Unkn= Unknown; NOS= not otherwise specified
Safety Study
A large multi-center study was performed in Canada where data were collected from patients who were treated with intravenous acetylcysteine for acetaminophen overdose between 1980 and 2005. This study evaluated 4709 adult cases and 1905 pediatric cases. The incidence of hypersensitivity reactions in adult (overall incidence 8%) and pediatric (overall incidence 10%) patients is presented in Tables 5 and 6.
Table 5. Distribution of reported hypersensitivity reactions in adult patients receiving intravenous acetylcysteine Reaction Incidence (%) n=4709 Urticaria/Facial Flushing 6.1% Pruritus 4.3% Respiratory Symptoms* 1.9% Edema 1.6% Hypotension 0.1% Anaphylaxis 0.1% *Respiratory symptoms are defined as presence of any of the following: cough, wheezing, stridor, shortness of breath, chest tightness, respiratory distress, or bronchospasm.
Table 6. Distribution of reported hypersensitivity reactions in pediatric patients receiving intravenous acetylcysteine Reaction Incidence (%) n=1905 Urticaria/Facial Flushing 7.6% Pruritus 4.1% Respiratory Symptoms* 2.2% Edema 1.2% Anaphylaxis 0.2% Hypotension 0.1% *Respiratory symptoms are defined as presence of any of the following: cough, wheezing, stridor, shortness of breath, chest tightness, respiratory distress, or bronchospasm.
6.2 Postmarketing Experience
Adverse Reactions from Observational Studies
Observational studies from published literature have described rates of hypersensitivity reactions identified by retrospective chart review in subjects receiving the two-bag regimen after adoption of this regimen as institutional policy in three non-US settings and one US setting compared to a historical control group that received the three-bag regimen.
Table 7 displays the incidence of hypersensitivity reactions in patients who received two-bag or three-bag regimens of intravenous acetylcysteine admitted between 2009 to 2020.
Table 7. Incidence of Hypersensitivity Reactions in Patients who Received Two-bag or Three-bag Regimens of Intravenous Acetylcysteine in Published Literature Study Hypersensitivity Reactions in Patients Receiving Two-Bag Regimen Hypersensitivity Reactions in Patients Receiving Three-Bag Regimen Study 1 (Three Danish hospitals) 4% (19/507) 16% (47/292) Study 2 (Four Australian hospitals) 5% (8/163) 14% (45/313) Study 3 (Nine Australian hospitals) 1% (17/1300) 7% (65/911) Study 4 (One US hospital) 19% (18/93) 23% (34/150) Adverse Reactions from Postmarketing Spontaneous Reports
The following adverse reactions have been identified during post-approval use of Acetylcysteine injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders: fatal anaphylaxis -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited published case reports and case series of pregnant women exposed to acetylcysteine during various trimesters are not sufficient to inform any drug associated risk. Delaying treatment of acetaminophen overdose may increase the risk of maternal or fetal morbidity and mortality [see Clinical Considerations].Reproduction studies in rats and rabbits following oral administration of acetylcysteine during the period of organogenesis at doses similar to the total intravenous dose (based on the body surface area) did not cause any adverse effects to the fetus. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Acetaminophen and acetylcysteine cross the placenta. Delaying treatment in pregnant women with acetaminophen overdose and potentially toxic acetaminophen plasma levels may increase the risk of maternal and fetal morbidity and mortality.
Data
Animal Data
Reproduction studies have been performed following administration of acetylcysteine during the period of organogenesis in rats at oral doses up to 2000 mg/kg/day (1.1 times the recommended total human intravenous dose of 300 mg/kg based on body surface area comparison) and in rabbits at oral doses up to 1000 mg/kg/day (1.1 times the recommended total human intravenous dose of 300 mg/kg based on body surface area comparison). No adverse developmental outcomes due to acetylcysteine were observed.
8.2 Lactation
Risk Summary
There are no data on the presence of acetylcysteine in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Acetylcysteine injection and any potential adverse effects on the breastfed child from Acetylcysteine injection or from the underlying maternal condition.
Clinical Considerations
Based on the pharmacokinetic data, acetylcysteine should be nearly completely cleared 30 hours after administration. Breastfeeding women may consider pumping and discarding their milk for 30 hours after administration.
8.4 Pediatric Use
Safety and effectiveness of Acetylcysteine injection in pediatric patients have not been established by adequate and well- controlled studies. Use of Acetylcysteine injection in pediatric patients 5 kg and greater is based on clinical practice [see Dosage and Administration (2.5)].
-
10 OVERDOSAGE
Fatal and life-threatening adverse events have been reported following acetylcysteine overdosage, including anaphylaxis, cerebral edema, and hemolytic-uremic syndrome (HUS). Stop acetylcysteine administration in the setting of suspected acetylcysteine overdosage and manage as clinically indicated.
Anaphylaxis, including cases with a fatal outcome, has been reported following acetylcysteine overdosage. Patients who experienced anaphylaxis following acetylcysteine overdosage often became symptomatic during the loading dose and experienced hypotension, rash, angioedema, bronchospasm, or respiratory distress. Cases of anaphylaxis with acetylcysteine overdosage also described coagulopathy, renal failure, or respiratory failure.
Cerebral edema, including cases with a fatal outcome, has been reported following acetylcysteine overdosage. Patients who experienced cerebral edema following acetylcysteine overdose presented with altered mental status, abnormal pupillary responses, seizures. Some cases of cerebral edema with acetylcysteine overdosage described brain herniation.
HUS has been reported following acetylcysteine overdosage. Patients who experienced HUS presented with microangiopathic hemolytic anemia, thrombocytopenia, and acute kidney injury.
Removal of acetylcysteine via hemodialysis has been reported in the literature outside of the context of acetylcysteine overdosage. Studies of hemodialysis in acetaminophen overdose report significant extracorporeal removal of acetylcysteine during hemodialysis. Contact the Poison Center (1-800-222-1222) for overdosage management recommendations for Acetylcysteine injection including considerations for hemodialysis.
-
11 DESCRIPTION
Acetylcysteine injection is an intravenous antidote for the treatment of acetaminophen overdose. Acetylcysteine is the nonproprietary name for the N-acetyl derivative of the naturally occurring amino acid, L-cysteine (N-acetyl-L cysteine,). The compound is a white crystalline powder, which melts in the range of 104° to 110°C and has a very slight odor.
The molecular formula of the compound is C 5H 9NO 3S, and its molecular weight is 163.2. Acetylcysteine has the following structural formula: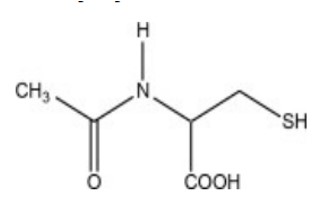
Acetylcysteine injection is supplied as a sterile solution in vials containing 20% w/v (200 mg/mL) acetylcysteine. The pH of the solution ranges from 6.0 to 7.5. Acetylcysteine injection contains the following inactive ingredients: sodium hydroxide (used for pH adjustment), Disodium Edetate Dihydrate and Water for Injection, USP.
The amount of sodium in Acetylcysteine injection is approximately 30 mg/mL. Because Acetylcysteine injection is administered based on a patient’s weight, the amount of sodium administered in a course of treatment will vary from approximately 225 mg to 4500 mg. The use of ½ normal saline will contribute approximately an additional 1770 mg of sodium per liter of diluent.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Acetylcysteine has been shown to reduce the extent of liver injury following acetaminophen overdose. Acetaminophen doses of 150 mg/kg or greater have been associated with hepatotoxicity. Acetylcysteine probably protects the liver by maintaining or restoring the glutathione levels, or by acting as an alternate substrate for conjugation with, and thus detoxification of, the reactive metabolite of acetaminophen.
12.3 Pharmacokinetics
After a single intravenous dose of acetylcysteine, the plasma concentration of total acetylcysteine declined in a poly-exponential decay manner with a mean terminal half-life (T1/2) of 5.6 hours.The mean clearance (CL) for acetylcysteine was 0.11 liter/hr/kg and renal CL constituted about 30% of the total CL.
Distribution:
The steady-state volume of distribution (Vd ss) following administration of an intravenous dose of acetylcysteine was 0.47 liter/kg. The protein binding of acetylcysteine ranges from 66 to 87%.
Elimination
Metabolism:
Acetylcysteine (i.e., N-acetylcysteine) is postulated to form cysteine and disulfides (N,N-diacetylcysteine and N acetylcysteine). Cysteine is further metabolized to form glutathione and other metabolites.
Excretion
After a single oral dose of [ 35S]-acetylcysteine 100 mg, between 13 to 38% of the total radioactivity administered was recovered in urine within 24 hours. In a separate study, renal clearance was estimated to be approximately 30% of total body clearance.
Specific Populations:
Hepatic Impairment:
Following a 600 mg intravenous dose of acetylcysteine to subjects with mild (Child Pugh Class A, n=1), moderate (Child-Pugh Class B, n=4) or severe (Child-Pugh Class C; n=4) hepatic impairment and 6 healthy matched controls, mean T1/2 increased by 80%. Also, the mean CL decreased by 30% and the systemic acetylcysteine exposure (mean AUC) increased 1.6-fold in subjects with hepatic impairment compared to subjects with normal hepatic function. These changes are not considered to be clinically meaningful.
Renal Impairment:
Hemodialysis may remove some of total acetylcysteine.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of acetylcysteine.
Mutagenesis
Acetylcysteine was not genotoxic in the Ames test or the in vivo mouse micronucleus test. It was, however, positive in the in vitro mouse lymphoma cell (L5178Y/TK+/-) forward mutation test.
Impairment of Fertility
In a fertility study of acetylcysteine in rats, intravenous administration of 1,000 mg/kg/day (0.5 times the recommended human dose of 300 mg/kg based on body surface area) caused a profound reduction of fertility in females, which was correlated with morphological changes in oocytes and severe impairment of implantation (18 of 20 mated females had no implantations). The reversibility of this effect was not evaluated. No effects on fertility were observed in female rats at intravenous doses up to 300 mg/kg/day (0.2 times the recommended human dose based on body surface area), or in male rats at intravenous doses up to 1,000 mg/kg/day. Mating was unaffected in this study.
In a reproduction study of acetylcysteine, male rats were treated orally for 15 weeks prior to mating and during the mating period. A slight non-dose related reduction in fertility was observed at oral doses of 500 and 1,000 mg/kg/day (0.3 and 0.5 times the recommended human intravenous dose, respectively, based on body surface area). Treatment of male rats with acetylcysteine at an oral dose of 250 mg/kg/day for 15 weeks (0.1 times the recommended human intravenous dose based on body surface comparison) did not affect fertility or general reproductive performance.
-
14 CLINICAL STUDIES
Loading Dose/Infusion Rate Study
A randomized, open-label, multi-center clinical study was conducted in Australia in patients with acetaminophen poisoning to compare the rates of hypersensitivity reactions between two rates of infusion for the intravenous acetylcysteine loading dose. One hundred nine subjects were randomized to a 15-minute infusion rate and seventy-one subjects were randomized to a 60 minute infusion rate. The loading dose was 150 mg/kg followed by a maintenance dose of 50 mg/kg over 4 hours and then 100 mg/kg over 16 hours. Of the 180 patients, 27% were male and 73% were female. Ages ranged from 15 to 83 years, with the mean age being 30 years ( +13.0).
A subgroup of 58 subjects (33 in the 15-minute infusion group; 25 in the 60-minute infusion group) was treated within 8 hours of acetaminophen ingestion. No hepatotoxicity occurred within this subgroup; however, with 95% confidence, the true hepatotoxicity rates could range from 0% to 9% for the 15-minute infusion group and from 0% to 12% for the 60-minute infusion group.
Observational Study
An open-label, observational database contained information on 1749 patients who sought treatment for acetaminophen overdose over a 16-year period. Of the 1749 patients, 65% were female, 34% were male and less than 1% was transgender. Ages ranged from 2 months to 96 years, with 72% of the patients falling in the 16- to 40-year-old age bracket. A total of 399 patients received acetylcysteine treatment. A post-hoc analysis identified 56 patients who (1) were at high or probable risk for hepatotoxicity (APAP greater than 150 mg/L at the four hours line according to the Australian nomogram) and (2) had a liver function test. Of the 53 patients who were treated with intravenous acetylcysteine (300 mg/kg intravenous acetylcysteine administered over 20-21 hours) within 8 hours, two (4%) developed hepatotoxicity (AST or ALT greater than 1000 U/L). Twenty-one of 48 (44%) patients treated with acetylcysteine after 15 hours developed hepatotoxicity. The actual number of hepatotoxicity outcomes may be higher than what is reported here. For patients with multiple admissions for acetaminophen overdose, only the first overdose treated with intravenous acetylcysteine was examined. Hepatotoxicity may have occurred in subsequent admissions.
Evaluable data were available from a total of 148 pediatric patients (less than 16 years of age) who were admitted for poisoning following ingestion of acetaminophen, of whom 23 were treated with intravenous acetylcysteine. There were no deaths of pediatric patients. None of the pediatric patients receiving intravenous acetylcysteine developed hepatotoxicity while two patients not receiving intravenous acetylcysteine developed hepatotoxicity. The number of pediatric patients is too small to provide a statistically significant finding of efficacy; however the results appear to be consistent to those observed for adults.
Three-Bag vs. Two-Bag Regimen Observational Study
A multi-center, observational, retrospective cohort study investigated rates of hypersensitivity reactions in 493 patients treated with a two-bag intravenous acetylcysteine regimen compared to 274 patients treated with a three-bag regimen in Denmark between January 2012 and December 2014. The two-bag regimen consisted of a 4 hour loading dose at 200 mg/kg followed by a 16 hour infusion at 100 mg/kg. The three-bag regimen used a 150 mg/kg loading dose administered over 15 minutes, followed by a second dose of 50 mg/kg over 4 hours and a final dose of 100 mg/kg for 16 hours. Patients in this study were treated with acetylcysteine regardless of plasma acetaminophen concentration. The median (range) serum acetaminophen concentration at presentation was <1 mcg/mL (0-353 mcg/mL) in the total study population, and only 12/767 patients (1.6%) had a serum acetaminophen level that would have warranted acetylcysteine treatment based on the revised Rumack-Mathew nomogram.
Hepatotoxicity was defined as peak ALT >1,000 U/L at any point during hospitalization. In this population that was low-risk for hepatotoxicity based on median acetaminophen level at presentation, no differences were observed in unadjusted hepatotoxicity rates between patients who received the two-bag regimen (4%, 20/493) compared to patients who received the three-bag regimen (4%, 11/274). However, this study has significant limitations and was not designed to establish non-inferiority.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Acetylcysteine injection (acetylcysteine) injection is available as a 20% solution (200 mg/mL) in 30 mL single-dose glass vials. Each single dose vial contains 6 g/30mL (200 mg/mL) of Acetylcysteine injection. Acetylcysteine injection is sterile and can be used for intravenous administration. It is available as follows:
- Carton of 4 x 30 mL Single-Dose Vials (NDC: 83270-001-04)
- 30 mL Single-Dose Vial (NDC: 83270-001-01)
Do not use previously opened vials for intravenous administration. Discard unused portion.
Note:The color of Acetylcysteine injection may turn from essentially colorless to a slight pink or purple once the stopper is punctured. The color change does not affect the quality of the product.
The stopper in the Acetylcysteine injection vial is formulated with a synthetic base-polymer and does not contain Natural Rubber Latex, Dry Natural Rubber, or blends of Natural Rubber.
Store unopened vials at controlled room temperature, 20°C to 25°C (68°F to 77°F) [See USP Controlled Room Temperature]
-
17 PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions
Advise patients and caregivers that hypersensitivity reactions related to administration and infusion may occur during and after Acetylcysteine injection treatment, including hypotension, wheezing, shortness of breath and bronchospasm [see Warnings and Precautions (5.1)].
For specific treatment information regarding the clinical management of acetaminophen overdose, please contact your regional poison center at 1-800-222-1222, or alternatively, a special health professional assistance line for acetaminophen overdose at 1-800-525-6115.
Mfd by:
OneSource Specialty Pharma Limited
Bengaluru, India.
Revised: September 2025
-
Principal Display Panel
PACKAGE LABEL - PRINCIPAL DISPLAY - Acetylcysteine 30 mL Single-Dose Vial Label
NDC 83270- 001-01
Acetylcysteine Injection
6 g/30 mL
(200 mg per mL)MUST BE FURTHER DILUTED PRIOR TO INTRAVENOUS USE.
Discard unused portion.
30 mL SterileSingle-Dose Vial
PACKAGE LABEL - PRINCIPAL DISPLAY - Acetylcysteine 30 mL Single-Dose Vial Carton Panel
NDC 83270- 001-04
Acetylcysteine Injection
6 g/30 mL
(200 mg per mL)MUST BE FURTHER DILUTED PRIOR TO INTRAVENOUS USE.
4 x 30 mL
SterileSingle-Dose Vials
-
INGREDIENTS AND APPEARANCE
ACETYLCYSTEINE
acetylcysteine injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 83270-001 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETYLCYSTEINE (UNII: WYQ7N0BPYC) (ACETYLCYSTEINE - UNII:WYQ7N0BPYC) ACETYLCYSTEINE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color pink (Clear colorless to to slight pink or purple color) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 83270-001-04 4 in 1 CARTON 04/26/2023 1 NDC: 83270-001-01 30 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA217182 04/26/2023 Labeler - ONESOURCE SPECIALTY PHARMA LIMITED (867530307) Registrant - Onesource Specialty Pte. Limited (599466600) Establishment Name Address ID/FEI Business Operations ONESOURCE SPECIALTY PHARMA LIMITED 867530307 manufacture(83270-001) , analysis(83270-001)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.