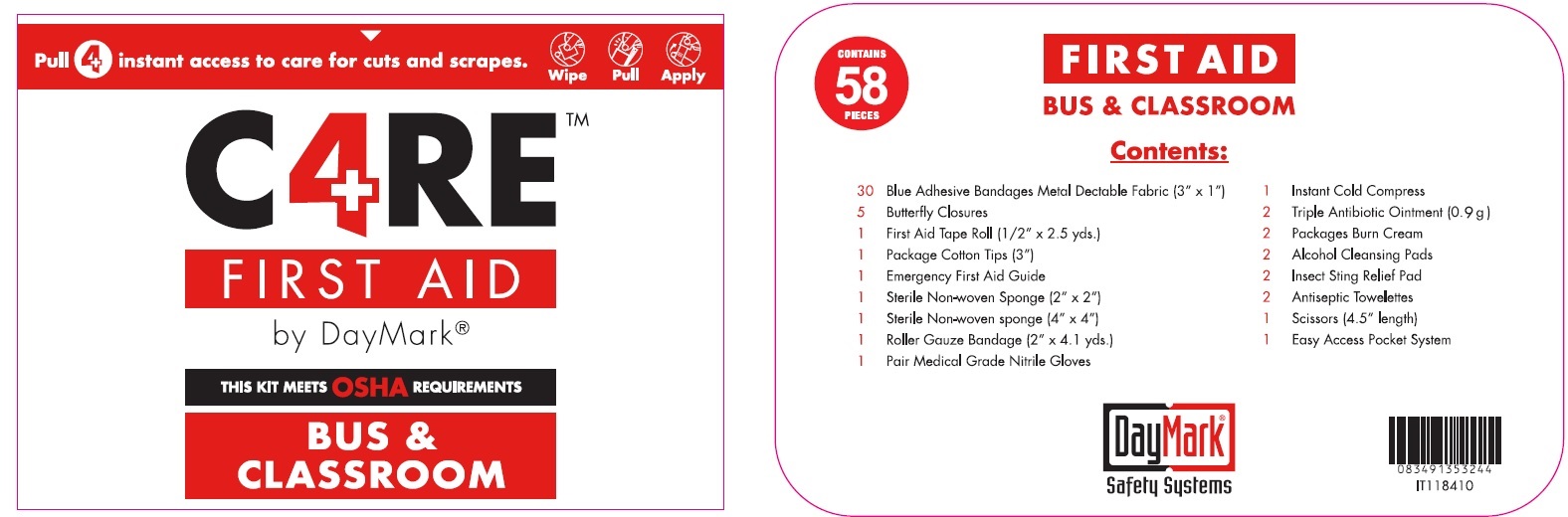
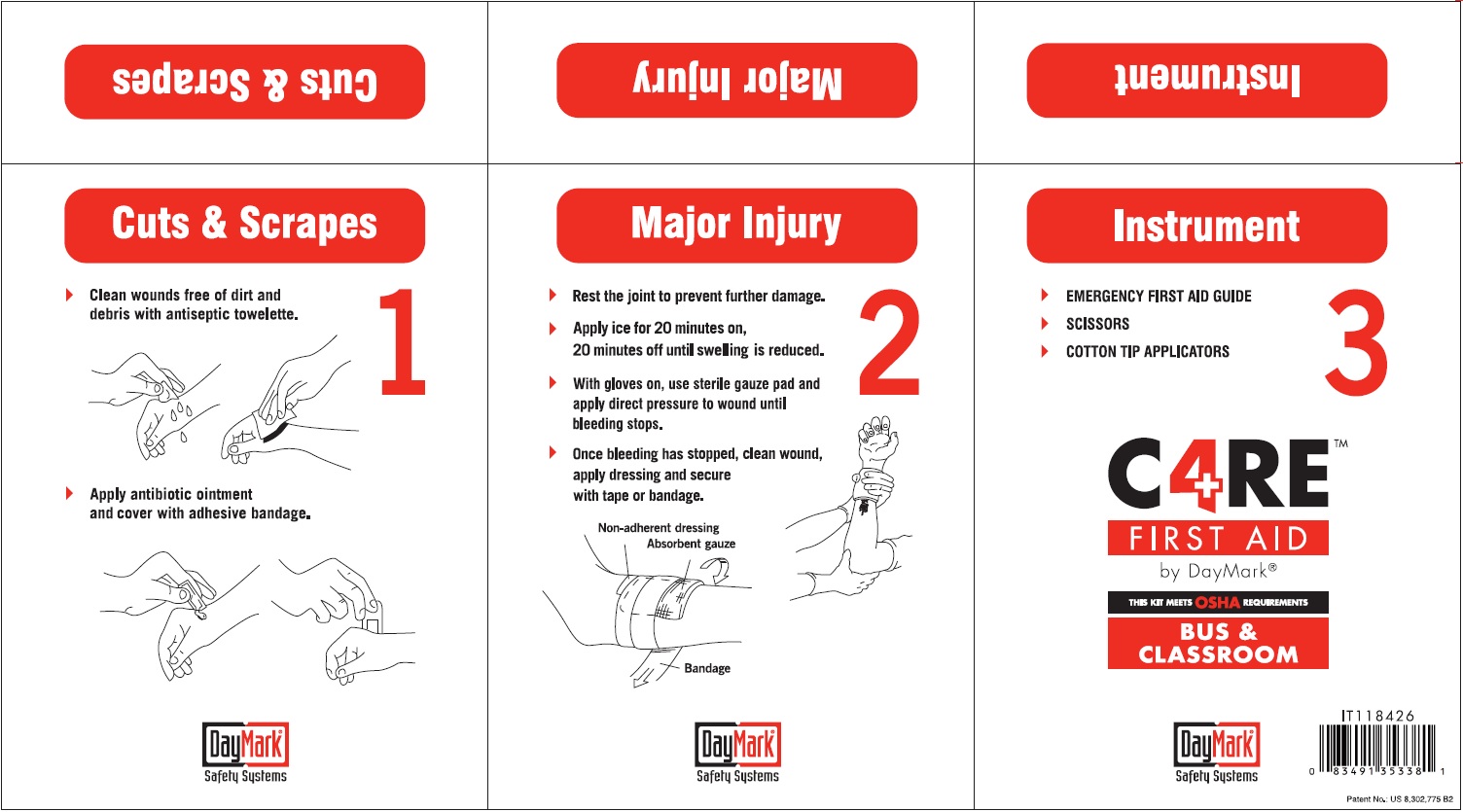
FAK CARE4 BUS AND SCHOOL YELLOW ORM D- bacitracin zinc, neomycin sulfate, polymyxin b sulfate, benzalkonium chloride, lidocaine hydrochloride, isopropyl alcohol, benzocaine, alcohol kit
FAK Care4 Bus and School Yellow ORM D by
Drug Labeling and Warnings
FAK Care4 Bus and School Yellow ORM D by is a Otc medication manufactured, distributed, or labeled by GFA Production (Xiamen) Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- First Aid Antibiotic Ointment, 0.9g (50814-007-01) Drug Facts
- Active ingredients (in each gram)
- Use
- Warnings
- Directions
- Other information
- Inactive ingredients
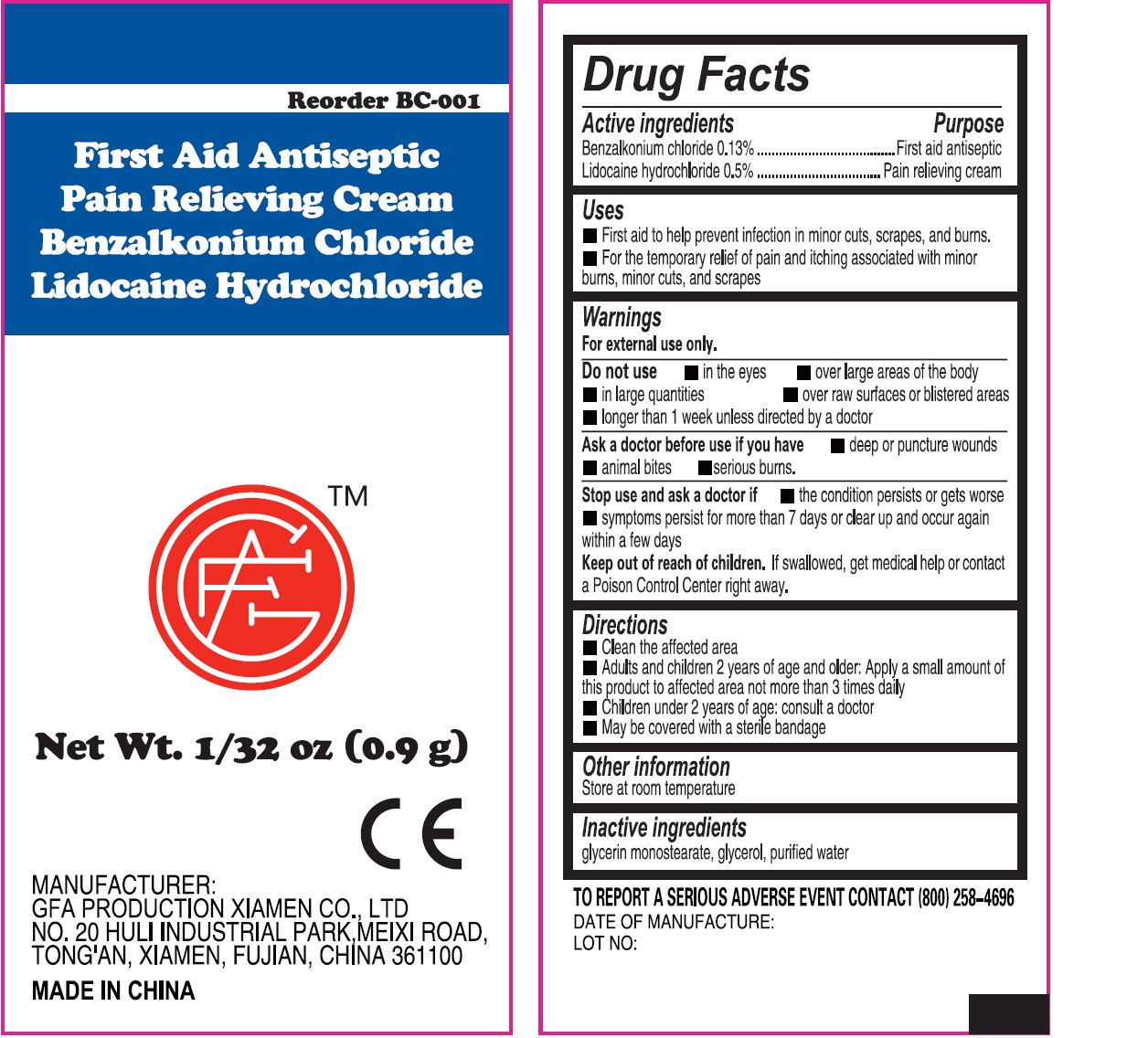
- First Aid Antiseptic Pain Relieving Cream, 0.9g (50814-009-01) Drug Facts
- Active ingredients
- Uses
-
Warnings
For external use only.
Do not use
in the eyes over large areas of the body in large quantities over raw surfaces or blistered areas longer than 1 week unless directed by a doctor
- Directions
- Other information
- Inactive ingredients
- Alcohol Cleansing Pad (50814-001-01) DRUG FACTS
- Active ingredient:
- Use:
- Warnings:
- Directions:
- Other information
- Inactive ingredient:
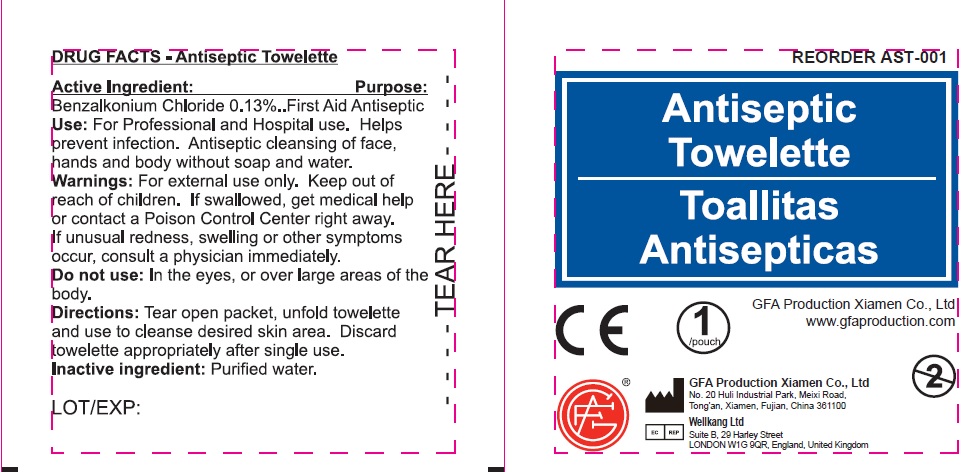
- Antiseptic Towelette (50814-002-01)DRUG FACTS
- Active Ingredient:
- Use:
- Warnings:
- Directions:
- Inactive ingredient:
- Insect Sting Relief Pad (52124-0008-1) DRUG FACTS
- Active Ingredient:
- Use:
- Warnings:
- Inactive Ingredients:
- Package Labeling:
- First Aid Antibiotic Ointment, 0.9g
- First Aid Antiseptic Pain Relieving Cream, 0.9g
- Alcohol Cleansing Pad
- Antiseptic Towelette
- Insect Sting Relief Pad
-
INGREDIENTS AND APPEARANCE
FAK CARE4 BUS AND SCHOOL YELLOW ORM D
bacitracin zinc, neomycin sulfate, polymyxin b sulfate, benzalkonium chloride, lidocaine hydrochloride, isopropyl alcohol, benzocaine, alcohol kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 50814-042 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50814-042-01 1 in 1 KIT 02/08/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BAG 0.9 g Part 2 1 PACKAGE 0.9 g Part 3 1 BAG 0.45 g Part 4 1 POUCH 0.45 g Part 5 1 POUCH 0.53 mL Part 1 of 5 FIRST AID ANTIBIOTIC
bacitracin zinc, neomycin sulfate, polymyxin b sulfate ointmentProduct Information Item Code (Source) NDC: 50814-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 KIT 1 NDC: 50814-007-01 0.9 g in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 02/08/2018 Part 2 of 5 FIRST AID ANTISEPTIC PAIN RELIEVING
benzalkonium chloride, lidocaine hydrochloride creamProduct Information Item Code (Source) NDC: 50814-009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 g LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 5 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 KIT 1 NDC: 50814-009-01 0.9 g in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 02/08/2018 Part 3 of 5 ALCOHOL CLEANSING
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 50814-039 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 700 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 KIT 1 NDC: 50814-039-01 1 in 1 BOX 1 0.45 g in 1 BAG; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 02/08/2018 Part 4 of 5 ANTISEPTIC TOWELETTE
benzalkonium chloride swabProduct Information Item Code (Source) NDC: 50814-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 KIT 1 NDC: 50814-002-01 1 in 1 BOX 1 0.45 g in 1 POUCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 02/08/2018 Part 5 of 5 INSECT STING RELIEF
benzocaine, alcohol swabProduct Information Item Code (Source) NDC: 50814-041 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 60 mg in 1 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 600 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 KIT 1 NDC: 50814-041-02 0.53 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 06/29/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 02/08/2018 Labeler - GFA Production (Xiamen) Co., Ltd. (421256261) Establishment Name Address ID/FEI Business Operations GFA Production (Xiamen) Co., Ltd. 421256261 manufacture(50814-042, 50814-007, 50814-009, 50814-039, 50814-002, 50814-041)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.