algeaSAN spa by sanPharma GmbH algeaSAN SPA
algeaSAN spa by
Drug Labeling and Warnings
algeaSAN spa by is a Other medication manufactured, distributed, or labeled by sanPharma GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ALGEASAN SPA- oyster shell calcium carbonate, crude, menaquinone 7, and cholecalciferol capsule
sanPharma GmbH
----------
algeaSAN SPA
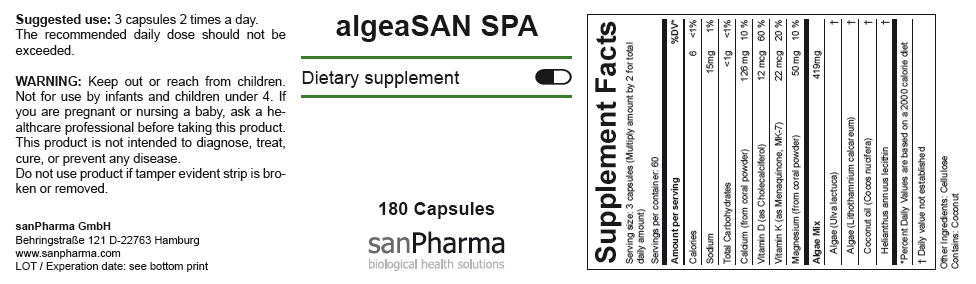
| Supplement Facts | ||
|---|---|---|
| Serving size: 3 capsules (Multiply amount by 2 for total daily amount) | ||
| Servings per container: 60 | ||
| Amount per serving | %DV* | |
|
|
||
| Calories | 6 | <1% |
| Sodium | 15mg | 1% |
| Total Carbohydrates | <1g | <1% |
| Calcium (from coral powder) | 126 mg | 10 % |
| Vitamin D (as Cholecalciferol) | 12 mcg | 60 % |
| Vitamin K (as Menaquinone, MK-7) | 22 mcg | 20 % |
| Magnesium (from coral powder) | 50 mg | 10 % |
| Algae Mix | 419mg | |
| Algae (Ulva lactuca) | † | |
| Algae (Lithothamnium calcareum) | † | |
| Coconut oil (Cocos nucifera) | † | |
| Helianthus annuus lecithin | † | |
WARNING
Keep out or reach from children. Not for use by infants and children under 4. If you are pregnant or nursing a baby, ask a healthcare professional before taking this product. This product is not intended to diagnose, treat, cure, or prevent any disease.
Do not use product if tamper evident strip is broken or removed.
| ALGEASAN SPA
oyster shell calcium carbonate, crude, menaquinone 7, and cholecalciferol capsule |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| scoring | 1 | |
| shape | ||
| size (solid drugs) | 22 mm | |
| Labeler - sanPharma GmbH (341409153) |
Revised: 10/2019
Document Id: b05433ca-9ab5-4a90-aaf0-797a52259e26
Set id: 3bb36a09-436c-4361-b691-9dda94db6a13
Version: 2
Effective Time: 20191015
sanPharma GmbH