FLYRCADO- flurpiridaz f-18 injection
Flyrcado by
Drug Labeling and Warnings
Flyrcado by is a Prescription medication manufactured, distributed, or labeled by GE Healthcare Inc., Pharmalogic Cincinnati, OH, Cardinal Health 414, LLC (Ft. Lauderdale, FL), Cardinal Health 418, Inc. (Aurora, CO), GE Healthcare Inc. (Medi-Physics, Inc. dba GE Healthcare), Cardinal Health 414, LLC (Beltsville, MD), Cardinal Health, Inc. (Houston, TX), Cardinal Health 414, LLC (Tampa, FL), Cardinal Health 414, LLC (Charlotte, NC), Cardinal Health 414, LLC (Colton, CA), Cardinal Health 414, LLC (New Orleans, LA), Cardinal Health 414, LLC (Phoenix, AZ), Cardinal Health 414, LLC (Dallas, TX), Cardinal Health, Inc. (Glendale Heights, IL), Cardinal Health 414, LLC (E. Hartford, CT), Cardinal Health 414, LLC (Seattle, WA), Pharmalogic New York City, LLC, Pharmalogic Los Angeles, LLC, PharmaLogic Salt Lake City, LLC, Hot Shots Nm, LLC, Pharmalogic Austin, LLC, Precision Nuclear, LLC (Johnson City, TN), Essential Isotopes, LLC. (Colombia, MO), Pharmalogic Colorado, LLC, SOFIE Co. dba SOFIE (Sanford, FL), SOFIE Co. dba SOFIE (Romeoville, IL), SOFIE Co. dba SOFIE (Sterling, VA), N-Molecular, Inc. dba SOFIE. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FLYRCADO™ safely and effectively. See full prescribing information for FLYRCADO.
FLYRCADO™ (flurpiridaz F 18) injection, for intravenous use
Initial U.S. Approval: 2024INDICATIONS AND USAGE
FLYRCADO is a radioactive diagnostic drug indicated for positron emission tomography (PET) myocardial perfusion imaging (MPI) under rest or stress (pharmacologic or exercise) in adult patients with known or suspected coronary artery disease (CAD) to evaluate for myocardial ischemia and infarction. (1)
DOSAGE AND ADMINISTRATION
- Administer FLYRCADO via intravenous injection. (2.2)
- When rest and stress imaging are conducted on the same day, the recommended administered activities are (2.2):
- Rest imaging: 93 MBq to 111 MBq (2.5 mCi to 3 mCi)
- Pharmacologic stress imaging: 222 MBq to 241 MBq (6 mCi to 6.5 mCi)
- Exercise stress imaging: 333 MBq to 352 MBq (9 mCi to 9.5 mCi)
- When rest and stress imaging are conducted over two days, the recommended rest and stress administered activities, for both pharmacologic and exercise stress, are 93 MBq to 111 MBq (2.5 mCi to 3 mCi). (2.2)
- See full prescribing information for radiation safety, preparation, administration, imaging, and radiation dosimetry information. (2.1, 2.2, 2.3, 2.4)
DOSAGE FORMS AND STRENGTHS
Injection: 190 MBq/mL to 2,050 MBq/mL (5 mCi/mL to 55 mCi/mL) of flurpiridaz F 18 at end of synthesis in a shielded multiple-dose vial with up to 30 mL fill volume (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Risk associated with exercise or pharmacologic stress: Serious adverse reactions such as myocardial infarction, arrhythmia, hypotension, broncho-constriction, stroke, and seizures may occur. Perform stress testing in the setting where cardiac resuscitation equipment and trained staff are readily available. When pharmacologic stress is selected, perform the procedure in accordance with the pharmacologic stress agent's prescribing information. (5.1)
- Radiation risks: Ensure safe handling to minimize radiation exposure to patients and health care providers. (5.2)
ADVERSE REACTIONS
Most common adverse reactions occurring during FLYRCADO PET MPI under rest and stress (pharmacologic or exercise) (incidence ≥ 2%) are dyspnea, headache, angina pectoris, chest pain, fatigue, ST segment changes, flushing, nausea, abdominal pain, dizziness, and arrhythmia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact GE HealthCare at 1-800-654-0118 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Lactation: Temporarily discontinue breastfeeding. A lactating woman should pump and discard breastmilk for at least 8 hours after FLYRCADO administration. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety – Drug Handling
2.2 Recommended Dosage, Preparation, and Administration Instructions
2.3 Image Acquisition Instructions
2.4 Radiation Dosimetry
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risks Associated with Exercise or Pharmacologic Stress
5.2 Radiation Risks
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
11.1 Chemical Characteristics
11.2 Physical Characteristics
11.3 External Radiation
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Overview of Clinical Studies
14.2 Suspected Coronary Artery Disease
14.3 Known or Suspected Coronary Artery Disease
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety – Drug Handling
Handle FLYRCADO with appropriate safety measures to minimize radiation exposure [see Warnings and Precautions (5.2)]. Use waterproof gloves and effective shielding, including lead-glass syringe shields, when handling and administering FLYRCADO.
Radioactive drugs should be used by or under the control of healthcare providers who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
2.2 Recommended Dosage, Preparation, and Administration Instructions
Recommended Dosage
- The recommended activity for each type of examination is presented in Table 1.
- Administer two doses by intravenous injection, one for rest imaging and one for stress imaging, using either pharmacologic or exercise stress, in either a 1-day or 2-day protocol.
- If performing a combined exercise/pharmacologic stress protocol, administer the recommended activity for pharmacologic stress.
- When rest and stress imaging are performed on the same day, the recommended minimum stress activity is 2-fold the rest activity for pharmacologic stress and 3-fold the rest activity for exercise stress to provide adequate image quality and obscure residual signal from the first acquisition.
- The recommended maximum total volume administered in one day is 6.1 mL.
Table 1 – Recommended Administered Activities of FLYRCADO in Adults for Rest and Stress Imaging Using Pharmacologic or Exercise Stress in a 1-Day or 2-Day Protocol Length of Protocol Activity for Rest Imaging Activity for Stress Imaging Pharmacologic Exercise 1 day 93 MBq to 111 MBq
(2.5 mCi to 3 mCi)222 MBq to 241 MBq
(6 mCi to 6.5 mCi)333 MBq to 352 MBq
(9 mCi to 9.5 mCi)2 days 93 MBq to 111 MBq
(2.5 mCi to 3 mCi)93 MBq to 111 MBq
(2.5 mCi to 3 mCi)93 MBq to 111 MBq
(2.5 mCi to 3 mCi)Patient Preparation
Instruct patients to drink water to ensure adequate hydration prior to administration of FLYRCADO and to continue drinking and voiding frequently during the first hours following administration to reduce radiation exposure [see Warnings and Precautions (5.2)].
Drug Preparation
- Use aseptic technique and radiation shielding to withdraw and administer FLYRCADO.
- Calculate the necessary volume to administer based on calibration time and activity using a suitably calibrated instrument.
- Visually inspect FLYRCADO for particulate matter and discoloration prior to administration and do not use if either is observed.
- Ensure the correct syringe is used and has adequate volume (at least 1 mL to 2 mL) so that the activity in the syringe can be administered without excessive dead volume loss.
- Measure patient dose using a dose calibrator immediately before administration.
- Do not use and discard FLYRCADO 8 hours after end of synthesis or when the activity falls below the activity requirement at the time of injection, whichever is earlier.
- FLYRCADO may be diluted aseptically with 0.9% Sodium Chloride Injection, USP. Diluted product should be used within 8 hours after end of synthesis or when the activity falls below the activity requirement at the time of injection, whichever is earlier.
- Dispose of unused product in a safe manner and in compliance with applicable regulations.
Administration Instructions
- Administer FLYRCADO via intravenous injection as a bolus lasting less than 10 seconds and immediately follow with a flush of 0.9% Sodium Chloride Injection, USP.
- The minimum time between rest and stress dose administration is:
- 30 minutes when using pharmacologic stress
- 60 minutes when using exercise stress
- When using pharmacologic stress, administer FLYRCADO at the time of peak vasodilation according to the prescribing information for the stress agent, using an intravenous port different from the one used for the stress agent.
- When using exercise stress, administer FLYRCADO after the patient reaches at least 85% of their age-predicted maximum heart rate or exhibits ischemic signs or symptoms. The patient should then continue to exercise for approximately 1 minute to 2 minutes after the injection. If the patient cannot achieve the target heart rate, the examination may be converted to pharmacologic stress.
2.3 Image Acquisition Instructions
For rest and stress imaging, image reconstruction should include attenuation correction.
Rest Imaging
Begin the PET acquisition 5 minutes after FLYRCADO administration and acquire images for 10 minutes using a scanner in 3D mode.
2.4 Radiation Dosimetry
Table 2 shows the estimated radiation absorbed doses per unit of injected activity.
Table 2 – Estimated Radiation Absorbed Doses in Organs/Tissues from Intravenous Administration of FLYRCADO in Adults Organ Absorbed Dose per Unit of Administered Activity
(mGy/MBq)Rest Pharmacologic Stress* Exercise Stress - * The pharmacologic stress agent used was adenosine.
Adrenals 0.016 0.016 0.014 Bone surfaces 0.019 0.019 0.02 Brain 0.025 0.022 0.011 Breasts 0.009 0.009 0.01 Gallbladder Wall 0.017 0.018 0.015 Gastrointestinal Tract Stomach Wall 0.04 0.033 0.024 Small Intestine Wall 0.013 0.012 0.014 Upper Large Intestine Wall 0.013 0.012 0.014 Lower Large Intestine Wall 0.012 0.011 0.014 Heart Wall 0.048 0.09 0.039 Kidneys 0.066 0.057 0.027 Liver 0.039 0.044 0.015 Lungs 0.011 0.012 0.012 Muscle 0.01 0.01 0.012 Ovaries 0.012 0.012 0.014 Pancreas 0.016 0.016 0.015 Red Marrow 0.016 0.018 0.015 Salivary Glands 0.035 0.076 0.007 Skin 0.008 0.008 0.009 Spleen 0.016 0.012 0.013 Testes 0.009 0.009 0.011 Thymus 0.011 0.012 0.013 Thyroid 0.032 0.036 0.014 Urinary Bladder Wall 0.023 0.021 0.016 Uterus 0.012 0.012 0.014 Total Body 0.012 0.012 0.012 Effective Dose (mSv/MBq) 0.019 0.019 0.015 The whole-body effective dose resulting from the administration of maximal activity of FLYRCADO of 111 MBq at rest, 241 MBq during pharmacological stress, and 352 MBq during exercise stress is, respectively, 2.1 mSv, 4.6 mSv, and 5.3 mSv. Under the same conditions, the absorbed dose to the target organ (heart wall) is 5.3 mGy, 22 mGy, and 14 mGy for each administered activity, respectively.
The use of a CT scan to calculate attenuation correction for the reconstruction of FLYRCADO PET images (as done in PET/CT imaging) will add radiation exposure.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risks Associated with Exercise or Pharmacologic Stress
Patients evaluated with exercise or pharmacologic stress may experience serious adverse reactions such as myocardial infarction, arrhythmia, hypotension, bronchoconstriction, stroke, and seizure. Perform stress testing in the setting where cardiac resuscitation equipment and trained staff are readily available. When pharmacologic stress is selected as an alternative to exercise, perform the procedure in accordance with the pharmacologic stress agent's prescribing information.
5.2 Radiation Risks
FLYRCADO contributes to a patient's overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk of cancer. Ensure safe handling to minimize radiation exposure to patients and health care providers [see Dosage and Administration (2.1, 2.4)]. Advise patients to hydrate before and after administration and to void frequently after administration.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Risks Associated with Exercise or Pharmacologic Stress [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of FLYRCADO was evaluated in 1,600 subjects in clinical studies, including 1,575 (98%) subjects with known or suspected coronary artery disease and 25 (2%) healthy subjects. All 1,600 subjects were dosed under rest conditions, with a mean dose of 102 MBq (2.8 mCi) FLYRCADO. A total of 1,568 (98%) subjects were also dosed under stress (exercise or pharmacologic) conditions, with a mean activity of 252 MBq (6.8 mCi) FLYRCADO by intravenous route. The demographic characteristics of the study population were 31% female, mean age 62 years (range 19 years to 90 years), 81% White, 11% Black or African American, 1% Asian, and 7% other or unreported race, and 10% Hispanic or Latino, 81% Not Hispanic or Latino, and 9% unreported ethnicity.
Stress testing procedures are associated with serious adverse reactions [see Warnings and Precautions (5.1)]. Adverse reactions occurring in ≥2% subjects receiving FLYRCADO during PET MPI under rest and stress (pharmacologic or exercise) are presented in Table 3.
Table 3. Adverse Reactions Reported in ≥2% of Subjects During FLYRCADO PET MPI Under Rest and Stress (Pharmacologic or Exercise) Adverse Reaction FLYRCADO PET MPI Under Rest and Stress
(Pharmacologic or Exercise)
N=1,600*
%- * Includes 32 subjects who received only one dose at rest.
Dyspnea 17 Headache 15 Angina pectoris 10 Chest pain 8 Fatigue 7 ST segment changes 6 Flushing 5 Nausea 4 Abdominal pain 4 Dizziness 4 Arrhythmia 4 Adverse reactions occurring during FLYRCADO PET MPI under rest and stress (pharmacologic or exercise) in <2% of subjects included diarrhea, palpitations, back pain, cardiac conduction disturbance, rash, dysgeusia, cough, hypotension, anxiety, vomiting, pruritus, bronchospasm, dry mouth, blood pressure elevation, syncope, and wheezing.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data on use of flurpiridaz F 18 in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes.
All radiopharmaceuticals, including FLYRCADO, have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of the radiation dose. If considering FLYRCADO administration to a pregnant woman, inform the patient about the potential for adverse pregnancy outcomes based on the radiation dose from flurpiridaz F 18 and the gestational timing of exposure.
FLYRCADO contains ethanol (a maximum daily dose of 337 mg anhydrous ethanol). The lower limit of safety for ethanol use during pregnancy has not been established. Published studies have demonstrated that ethanol is associated with fetal harm including central nervous system abnormalities, behavioral disorders, and impaired intellectual development. If considering FLYRCADO administration to a pregnant woman, inform the patient about the potential for adverse pregnancy outcomes associated with ethanol exposure during pregnancy.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of flurpiridaz F 18 or its metabolites in human milk or animal milk, the effects on the breastfed infant, or the effects on milk production. Based on clinical guidelines, exposure of FLYRCADO to a breastfed infant can be minimized by advising a lactating woman to temporarily discontinue breastfeeding and to pump and discard breast milk for a minimum of 8 hours after administration of FLYRCADO. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for FLYRCADO and any potential adverse effects on the breastfed child from FLYRCADO or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of FLYRCADO in pediatric patients have not been established.
8.5 Geriatric Use
Of 1,600 subjects in clinical studies of FLYRCADO, 720 (45%) were 65 years of age and over and 181 (11%) were 75 years of age or older. No overall differences in safety or effectiveness of FLYRCADO have been observed between patients 65 years of age and older and younger adult patients.
-
11 DESCRIPTION
11.1 Chemical Characteristics
FLYRCADO (flurpiridaz F 18) injection is a radioactive diagnostic drug for intravenous use. The molecular formula of flurpiridaz F 18 is C18H22Cl18FN2O3, the molecular mass is 367.8, and the structural formula is:
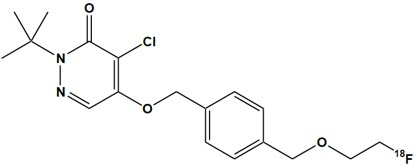
Chemically, flurpiridaz F 18 is 2-tert-butyl-4-chloro-5-[[4-(2-(18F)fluoranylethoxymethyl)phenyl]methoxy]pyridazin-3-one.
FLYRCADO is a sterile, preservative-free, non-pyrogenic, clear, colorless to yellow radioactive solution. Each mL contains 190 MBq to 2,050 MBq (5 mCi to 55 mCi) of flurpiridaz F 18 at end of synthesis, up to 2.3 mcg flurpiridaz, and the following inactive ingredients: 45 mg hydroxypropyl-β-cyclodextrin (as a solubilizer and co-radiostabilizer), 35 mg L-(+)-ascorbic acid (as a radiostabilizer), 8.2 mg sodium hydroxide, and 55.2 mg anhydrous ethanol, in water for injection. The pH of the solution is between 5.5 and 8.
11.2 Physical Characteristics
Fluorine-18 decays by positron (β+) emission and has a half-life of 109.8 minutes. The principal photons useful for diagnostic imaging are the 511 keV gamma photons, resulting from the interaction of the emitted positron with an electron. Principal emission data for fluorine-18 are shown in Table 4.
Table 4 – Principal Radiation Emission Data for Fluorine-18 Radiation/Emission % per Disintegration Mean Energy (keV) Positron 96.7 249.8 Gamma 193.5 511 11.3 External Radiation
The point source air-kerma rate constant for fluorine-18 is 3.74E-17 Gy m2/(Bq s); this coefficient was formerly defined as the specific gamma-ray constant of 5.7 R/hr/mCi at 1 cm. The first half-value thickness of lead (Pb) for fluorine-18 gamma rays is approximately 6 mm. The relative reduction of radiation emitted by fluorine-18 that results from various thicknesses of lead shielding is shown in Table 5. The use of about 8 cm of Pb will decrease the radiation transmission (i.e., exposure) by a factor of about 10,000.
Table 5 - Radiation Attenuation of 511 keV Gamma Rays by Lead Shielding Shielding Thickness cm of Lead (Pb) Coefficient of Attenuation 0.6 0.5 2 0.1 4 0.01 6 0.001 8 0.0001 -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Flurpiridaz F 18 is an analog of the mitochondrial complex 1 (MC-1) inhibitor, pyridaben. Flurpiridaz F 18 is extracted by the myocardium proportional to the blood flow and binds to heart tissue that has biologically active mitochondria. Therefore, radioactivity in viable myocardium is higher than in infarcted tissue.
12.2 Pharmacodynamics
The relationship between flurpiridaz F 18 plasma concentrations and successful imaging was not explored in clinical trials.
12.3 Pharmacokinetics
The pharmacokinetics of flurpiridaz F 18 were evaluated in healthy subjects. Blood radioactivity peaked at 2.3 minutes with blood clearance followed by a rise and plateau at around 3% of the 296 MBq (8 mCi) flurpiridaz F 18 intravenous dose until 7 hours post administration. The fluorine-18 radioactivity in blood during the first 15-minutes post administration was associated with flurpiridaz while it was associated with flurpiridaz metabolites thereafter.
Distribution
Flurpiridaz F 18 distributes to the liver (19% of injected activity), kidneys (9%), brain (8%), and heart wall (3%) about 10 minutes post-dose. The heart wall radioactivity was retained for 1 hour after administration.
Specific Populations
No clinically significant differences in the pharmacokinetics of flurpiridaz F 18 were observed for age, sex, body mass index, diabetic status, mild hepatic impairment (Child Pugh A), or renal impairment (eGFR ≥19 to 89 mL/min). The effect of moderate to severe hepatic impairment (Child Pugh B and C) or end stage renal disease on flurpiridaz F 18 pharmacokinetics has not been evaluated.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Overview of Clinical Studies
The safety and effectiveness of FLYRCADO were evaluated in two prospective, multicenter, open-label clinical studies in adults with either suspected coronary artery disease (CAD) (Study 1: NCT03354273) or known or suspected CAD (Study 2: NCT01347710).
Subjects received two injections of FLYRCADO: one at rest and one during stress [see Dosage and Administration (2.2, 2.3)]. For the FLYRCADO stress injection, subjects received either a pharmacologic stress agent or engaged in exercise stress. PET myocardial perfusion imaging (MPI) was performed at both rest and stress using cardiac gating and low-dose CT attenuation correction. Subjects also received rest and stress SPECT MPI using technetium Tc 99m sestamibi or technetium Tc 99m tetrofosmin on a different day from the PET MPI. The stress modality was to be the same for PET and SPECT.
Stress and rest images were displayed side-by-side for the assessment of perfusion and wall motion abnormalities. Three qualified readers, blinded to clinical data, performed independent assessment of each subject's rest and stress images, with each recording an overall qualitative diagnosis of normal, ischemia, ischemia plus scar, or scar. For analyses of sensitivity and specificity, normal was considered image negative and all other diagnoses were considered image positive.
14.2 Suspected Coronary Artery Disease
Study 1 evaluated the sensitivity and specificity of FLYRCADO PET MPI for the detection of significant CAD in subjects with suspected CAD who were scheduled for invasive coronary angiography (ICA).
A total of 578 subjects were evaluable for effectiveness, having rest and stress imaging and evaluable truth standard data. Subjects ranged in age from 26 years to 88 years, with a mean age of 64 years. A total of 188 (33%) were female, and 473 (82%) were White, 35 (6%) were Black or African American, 6 (1%) were Asian, and 64 (11%) were other races or not reported. In addition, 79 subjects (14%) reported Hispanic/Latino ethnicity. Pharmacologic stress was performed in 83% of subjects and exercise stress in 17% of subjects.
The sensitivity and specificity of FLYRCADO PET MPI for detection of significant CAD, defined as the presence of significant stenosis in at least one major epicardial coronary artery or major branch by quantitative coronary angiography (QCA) are reported in Table 6. Results for both ≥50% stenosis and a secondary analysis using ≥70% stenosis as the threshold for significant CAD are shown.
Table 6. Diagnostic Performance of FLYRCADO PET MPI in Study 1 (N = 578*) ≥50% Stenosis Reference Standard ≥70% Stenosis Reference Standard Reader Sensitivity (95% CI)
N=249Specificity (95% CI)
N=329Sensitivity (95% CI)
N=127Specificity (95% CI)
N=449Abbreviations: CI = confidence interval, MPI = myocardial perfusion imaging - * Includes 12 subjects who had reference standard images categorized as uninterpretable by the central reader but were forced to be diagnosed as positive or negative (2 subjects as positive and 10 subjects as negative).
Reader 1 77%
(72%, 82%)66%
(61%, 71%)91%
(86%, 96%)58%
(54%, 63%)Reader 2 74%
(68%, 79%)70%
(65%, 75%)87%
(82%, 93%)62%
(58%, 67%)Reader 3 89%
(85%, 93%)53%
(47%, 58%)97%
(94%, 100%)44%
(39%, 49%)From a blinded re-evaluation of 60 randomly selected PET MPI images presented during the main reading sessions, intra-reader kappa ranged from 0.71 to 0.93 for the three readers.
Using a 50% stenosis threshold, sensitivity of SPECT MPI was 61% to 76% for the three readers, with the lower bound of the 95% confidence intervals ranging from 55% to 70%, and specificity of SPECT MPI was 51% to 65%, with the lower bound of the 95% confidence intervals ranging from 46% to 60%.
14.3 Known or Suspected Coronary Artery Disease
Study 2 evaluated the sensitivity and specificity of FLYRCADO PET MPI for the detection of significant CAD in subjects with known or suspected CAD who had ICA without intervention within 60 days prior to imaging or were scheduled for ICA.
A total of 755 subjects were evaluable for effectiveness, having rest and stress imaging for FLYRCADO PET and evaluable truth standard data. Subjects ranged in age from 36 years to 90 years, with a mean age of 63 years. A total of 235 (31%) were female, and 619 (82%) were White, 101 (13%) were Black or African American, 8 (1%) were Asian, and 24 (4%) were other races or not reported. Pharmacologic stress was performed in 71% of subjects and exercise stress in 29% of subjects.
The sensitivity and specificity of FLYRCADO PET MPI for the detection of significant CAD, defined as the presence of significant stenosis in at least one major epicardial coronary artery or major branch by QCA or as history of myocardial infarction are reported in Table 7. Results for both ≥50% stenosis and a secondary analysis using a threshold of ≥70% stenosis for significant CAD are shown.
Table 7. Diagnostic Performance of FLYRCADO PET MPI in Study 2 (N = 755) ≥50% Stenosis or Confirmed MI Reference Standard ≥70% Stenosis or Confirmed MI Reference Standard Reader Sensitivity (95% CI)
N=352Specificity (95% CI)
N=403Sensitivity (95% CI)
N=245Specificity (95% CI)
N=510Abbreviations: CI = confidence interval, MI = myocardial infarction, MPI = myocardial perfusion imaging Reader 1 73%
(68%, 78%)73%
(68%, 77%)82%
(77%, 87%)68%
(63%, 71%)Reader 2 63%
(57%, 67%)86%
(82%, 89%)72%
(66%, 78%)80%
(77%, 83%)Reader 3 77%
(72%, 80%)66%
(62%, 71%)85%
(80%, 89%)61%
(57%, 66%)From a blinded re-evaluation of a randomly selected 10% of PET MPI images presented during the main reading sessions, intra-reader agreement ranged from 90% to 95% for the three readers.
Using a 50% stenosis threshold, sensitivity of SPECT MPI was 43% to 58% for the three readers, with the lower bound of the 95% confidence intervals ranging from 38% to 53%, and specificity for SPECT MPI was 80% to 92%, with the lower bound of the 95% confidence intervals ranging from 76% to 89%.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
FLYRCADO (flurpiridaz F 18) injection is a clear, colorless to yellow solution containing 190 MBq/mL to 2,050 MBq/mL (5 mCi/mL to 55 mCi/mL) of flurpiridaz F 18 at end of synthesis, supplied in a shielded multiple-dose vial (NDC: 0407-8787-01) with up to 30 mL fill volume.
Storage and Handling
Store FLYRCADO at 2°C to 30°C (36°F to 86°F); excursions to -20°C (-4°F) (up to 2 hours) or to 50°C (122°F) (up to 8 hours) may be permitted. Store FLYRCADO within radiation shielding. The product does not contain a preservative.
Do not use and discard FLYRCADO 8 hours after end of synthesis or when the activity falls below the radioactivity requirement at the time of injection, whichever is earlier. The expiration date and time are provided on the shield label.
Dispose of the product in accordance with all federal, state, and local laws and institutional requirements.
This preparation is for use by persons licensed by the Nuclear Regulatory Commission or the relevant regulatory authority of an Agreement State.
-
17 PATIENT COUNSELING INFORMATION
Adequate Hydration
Instruct patients to drink water to ensure adequate hydration prior to administration of FLYRCADO and to continue drinking and voiding frequently during the first hours following administration to reduce radiation exposure [see Warnings and Precautions (5.2)].
Pregnancy
Inform pregnant women of the risks of fetal exposure to radiation dose if they undergo a radionuclide procedure and the potential risk of exposure to ethanol which is present in FLYRCADO [see Use in Specific Populations (8.1)].
Lactation
Advise a lactating woman to temporarily discontinue breastfeeding and to pump and discard breast milk for at least 8 hours after FLYRCADO administration to minimize radiation exposure to a breastfed infant [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
-
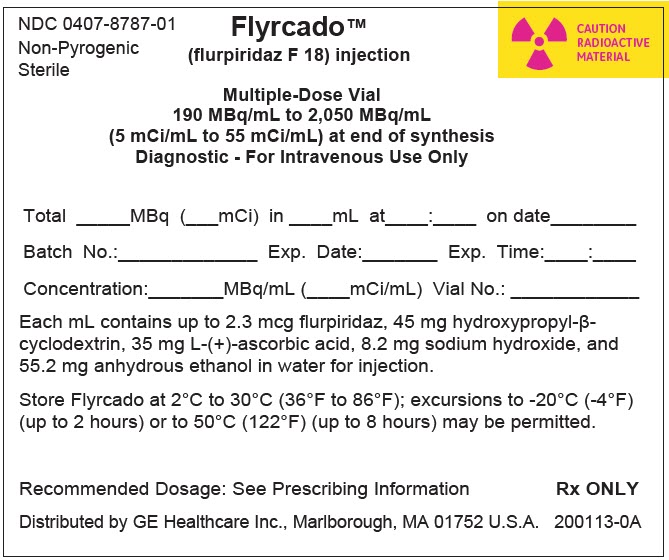
PRINCIPAL DISPLAY PANEL - 30 mL Vial Label
NDC: 0407-8787-01
Multiple-Dose Vial
Non-Pyrogenic
SterileFlyrcado™
(flurpiridaz F 18) injection
190 MBq/mL to 2,050 MBq/mL
(5 mCi/mL to 55 mCi/mL) at end of synthesis
Diagnostic - For Intravenous Use OnlyCAUTION
RADIOACTIVE
MATERIALTotal: ______MBq (____mCi) in_____mL at _____:_____ on date ___________
Concentration: ____MBq/mL (____mCi/mL) Exp Date_________ Exp Time__________
Batch No.: _______________ Date: ________________ Vial No.: __________
Store FLYRCADO at 2°C to 30°C (36°F to 86°F); excursions to -20°C (-4°F) (up to 2 hours) or to 50°C
(122°F) (up to 8 hours) may be permitted.
Recommended Dosage: See Prescribing InformationDistributed by GE Healthcare Inc., Arlington Heights, IL 60004, U.S.A.
Rx ONLY
200112-0B
-
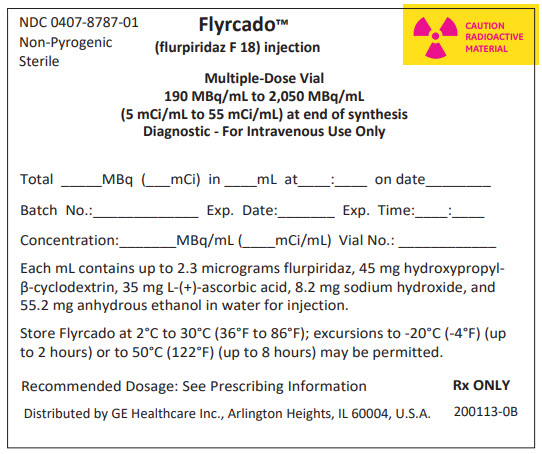
PRINCIPAL DISPLAY PANEL - 30 mL Shield Label
NDC: 0407-8787-01
Non-Pyrogenic
SterileFlyrcado™
(flurpiridaz F 18) injectionCAUTION
RADIOACTIVE
MATERIALMultiple-Dose Vial
190 MBq/mL to 2,050 MBq/mL
(5 mCi/mL to 55 mCi/mL) at end of synthesis
Diagnostic - For Intravenous Use OnlyTotal _____MBq (___mCi) in ____mL at____:____ on date________
Batch No.:_____________ Exp. Date:_______ Exp. Time:____:____
Concentration:_______MBq/mL (____mCi/mL) Vial No.: ____________
Each mL contains up to 2.3 micrograms flurpiridaz, 45 mg hydroxypropyl-
β-cyclodextrin, 35 mg L-(+)-ascorbic acid, 8.2 mg sodium hydroxide, and
55.2 mg anhydrous ethanol in water for injection.Store Flyrcado at 2°C to 30°C (36°F to 86°F); excursions to -20°C (-4°F) (up
to 2 hours) or to 50°C (122°F) (up to 8 hours) may be permitted.Recommended Dosage: See Prescribing Information
Distributed by GE Healthcare Inc., Arlington Heights, IL 60004, U.S.A.
Rx ONLY
200113-0B
-
INGREDIENTS AND APPEARANCE
FLYRCADO
flurpiridaz f-18 injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0407-8787 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLURPIRIDAZ F-18 (UNII: TY3V24C029) (FLURPIRIDAZ F-18 - UNII:TY3V24C029) FLURPIRIDAZ F-18 55 mCi in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) ASCORBIC ACID (UNII: PQ6CK8PD0R) HYDROXYPROPYL BETADEX (UNII: 1I96OHX6EK) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (Colorless to yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0407-8787-01 30 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 02/19/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215168 02/19/2025 Labeler - GE Healthcare Inc. (053046579) Establishment Name Address ID/FEI Business Operations Pharmalogic Cincinnati, OH 118408248 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Cardinal Health 414, LLC (Ft. Lauderdale, FL) 964767722 MANUFACTURE(0407-8787) , POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Cardinal Health 418, Inc. (Aurora, CO) 149029253 ANALYSIS(0407-8787) Establishment Name Address ID/FEI Business Operations GE Healthcare Inc. (Medi-Physics, Inc. dba GE Healthcare) 095263729 ANALYSIS(0407-8787) Establishment Name Address ID/FEI Business Operations Cardinal Health 414, LLC (Beltsville, MD) 964767771 MANUFACTURE(0407-8787) , POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Cardinal Health, Inc. (Houston, TX) 826800364 MANUFACTURE(0407-8787) , POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Cardinal Health 414, LLC (Tampa, FL) 964768340 MANUFACTURE(0407-8787) , POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Cardinal Health 414, LLC (Charlotte, NC) 964768373 MANUFACTURE(0407-8787) , POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Cardinal Health 414, LLC (Colton, CA) 964767656 MANUFACTURE(0407-8787) , POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Cardinal Health 414, LLC (New Orleans, LA) 080130462 MANUFACTURE(0407-8787) , POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Cardinal Health 414, LLC (Phoenix, AZ) 833114734 MANUFACTURE(0407-8787) , POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Cardinal Health 414, LLC (Dallas, TX) 964768282 MANUFACTURE(0407-8787) , POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Cardinal Health, Inc. (Glendale Heights, IL) 033231923 MANUFACTURE(0407-8787) , POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Cardinal Health 414, LLC (E. Hartford, CT) 964768233 MANUFACTURE(0407-8787) , POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Cardinal Health 414, LLC (Seattle, WA) 964768126 MANUFACTURE(0407-8787) , POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Pharmalogic New York City, LLC 118410433 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Pharmalogic Los Angeles, LLC 119024924 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations PharmaLogic Salt Lake City, LLC 119319723 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Hot Shots Nm, LLC 622386605 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Pharmalogic Austin, LLC 118431835 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Precision Nuclear, LLC (Johnson City, TN) 879283633 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Essential Isotopes, LLC. (Colombia, MO) 010753961 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Pharmalogic Colorado, LLC 117608150 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations SOFIE Co. dba SOFIE (Sanford, FL) 006320902 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations SOFIE Co. dba SOFIE (Romeoville, IL) 032324142 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations SOFIE Co. dba SOFIE (Sterling, VA) 079854636 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations N-Molecular, Inc. dba SOFIE 079932640 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations N-Molecular, Inc. dba SOFIE (Oakwood Village, OH) 079932600 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations SOFIE Co. dba SOFIE (Totowa, NJ) 928455851 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations SOFIE Co. dba SOFIE (Kansas City, MO) 829109441 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations Wisconsin Medical Radiopharmacy, LLC 117749860 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787) Establishment Name Address ID/FEI Business Operations SOFIE Co. dba SOFIE (Gilroy, CA) 832599976 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(0407-8787)
Trademark Results [Flyrcado]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 FLYRCADO 97709172 not registered Live/Pending |
GE Healthcare Limited 2022-12-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.