CETIRIZINE HYDROCHLORIDE- cetirizine hydrochloride tablet, chewable
cetirizine hydrochloride by
Drug Labeling and Warnings
cetirizine hydrochloride by is a Otc medication manufactured, distributed, or labeled by Jubilant Cadista Pharmaceuticals Inc., Jubilant Generics Limited, Jubilant Generics Limited Roorkee. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient ( in each chewable tablet )
- Purpose
- Uses:
- Warnings:
- Do not use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
- Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
- Ask a doctor or pharmacist before use if you are taking tranquilizers or sedatives.
- When using this product:
- Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
- If pregnant or breast-feeding:
- Keep out of reach of children.
-
Directions:
● may be taken with and without water
For Cetirizine hydrochloride chewable tablets 5 mg
adults and children 6 years and over
1 to 2 tablets once daily depending upon severity of symptoms; do not take more than 2 tablets in 24 hours.
adults 65 years and over
1 tablet once a day; do not take more than 1 tablet in 24 hours.
children under 6 years of age
ask a doctor
consumers with liver or kidney disease
ask a doctor
For Cetirizine hydrochloride chewable tablets 10 mg
adults and children 6 years and over
one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms.
adults 65 years and over
ask a doctor
children under 6 years of age
ask a doctor
consumers with liver or kidney disease
ask a doctor
Other information:
-
store between 20º to 25º C (68º to 77º F)
-
Phenylketonurics: Contains 1.68 mg Phenylalanine (a component of Aspartame) per 5 mg
-
Phenylketonurics: Contains 3.36 mg Phenylalanine (a component of Aspartame) per 10 mg
-
do not use if carton is opened or if blister unit is broken.
-
see bottom panel for lot number and expiration date.
-
-
Inactive ingredients:
acesulfame potassium, artificial and natural flavors, aspartame, betadex, colloidal silicon dioxide, croscarmellose sodium, dl-alpha-tocopherol, ethyl cellulose, FD&C yellow # 6 aluminum lake, fumaric acid, hypromellose, magnesium stearate, mannitol, maltodextrin, microcrystalline cellulose and talc.
- Questions? call 1-800-313-4623
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
CADISTA NDC: 59746-285-32
Children's
Cetirizine Hydrochloride Chewable Tablets 5 mg
Antihistamine
ALLERGY
Phenylketonurics: contains 1.68 mg Phenylalanine (a component of Aspartame) per 5 mg tablets.
Indoor & Outdoor Allergies
24 hour Relief of
● Sneezing
● Running Nose
● Itchy,Watery Eyes
● Itchy Throat or Nose
TAMPER EVIDENT : Do not use if blister unit is broken or torn.
6 yrs. & older
5 mg each
30 Chewable Tablets Orange Chewables
5 mg each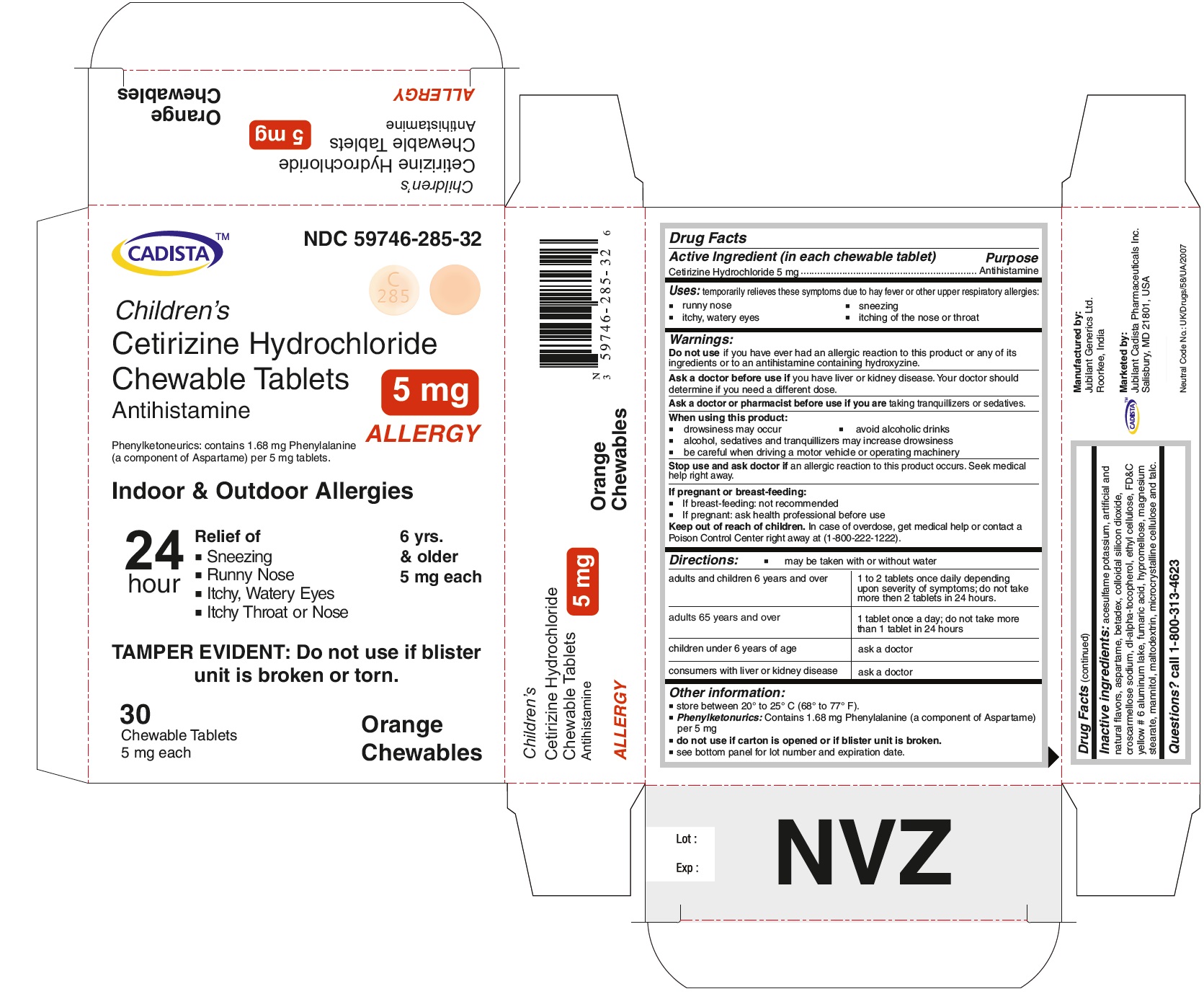
Cetirizine Hydrochloride Chewable Tabelets 5 mg Allergy
CADISTA NDC: 59746-286-32
Children's
Cetirizine Hydrochloride Chewable Tablets 10 mg
Antihistamine
ALLERGY
Phenylketonurics: contains 3.36 mg Phenylalanine ( a component of Aspartame) per 10 mg tablets.
Indoor & Outdoor Allergies
24 hour Relief of
● Sneezing
● Running Nose
● Itchy,Watery Eyes
● Itchy Throat or Nose
TAMPER EVIDENT : Do not use if blister unit is broken or torn.
6 yrs. & older
10 mg each
30 Chewable Tablets Orange Chewables
10 mg each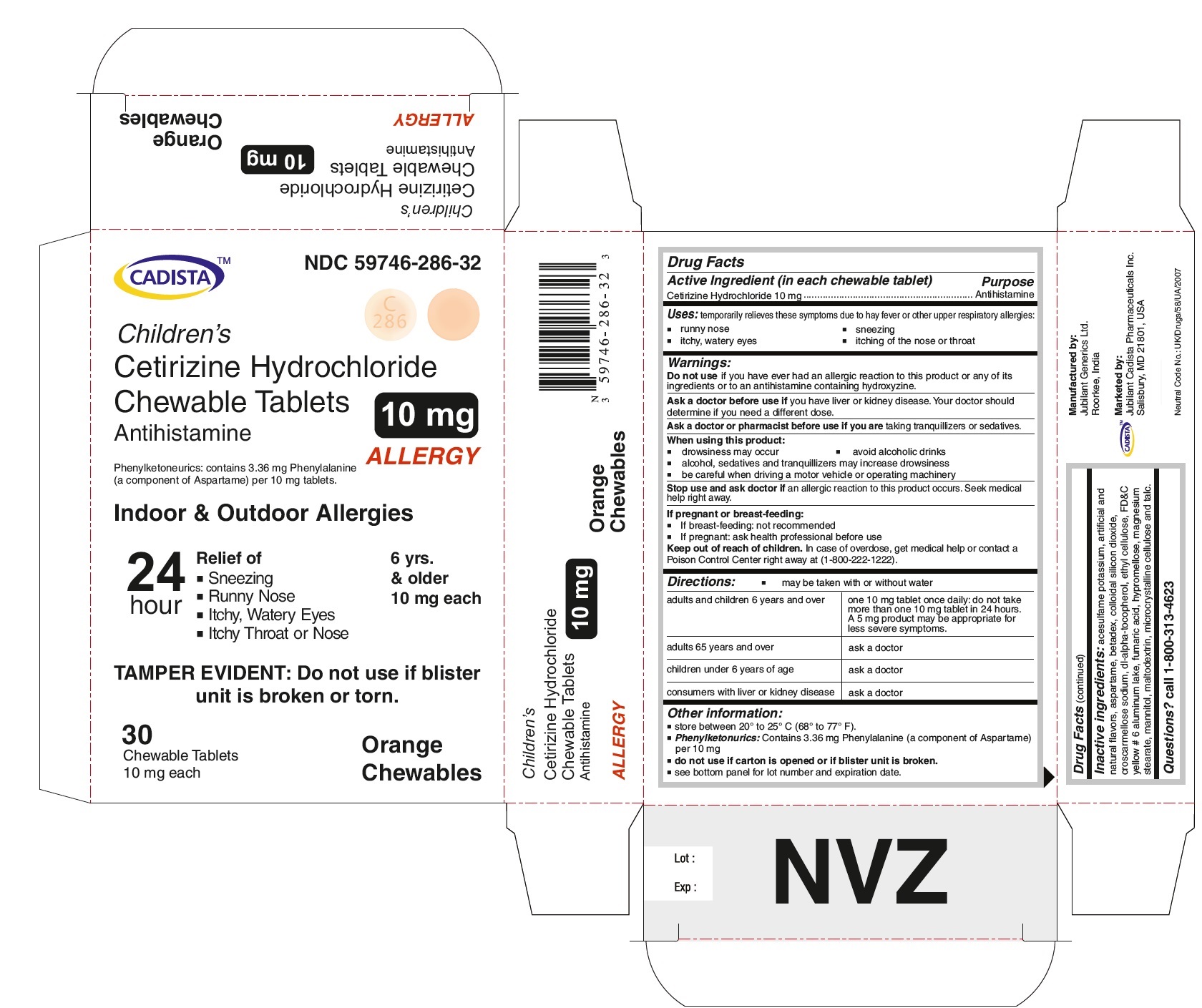
Cetirizine Hydrochloride Chewable Tablets 10 mg Allergy
-
INGREDIENTS AND APPEARANCE
CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59746-285 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Cetirizine Hydrochloride (UNII: 64O047KTOA) (Cetirizine - UNII:YO7261ME24) Cetirizine Hydrochloride 5 mg Inactive Ingredients Ingredient Name Strength Acesulfame Potassium (UNII: 23OV73Q5G9) Aspartame (UNII: Z0H242BBR1) Betadex (UNII: JV039JZZ3A) Silicon Dioxide (UNII: ETJ7Z6XBU4) Croscarmellose Sodium (UNII: M28OL1HH48) .alpha.-tocopherol, Dl- (UNII: 7QWA1RIO01) Ethylcelluloses (UNII: 7Z8S9VYZ4B) Fd&c Yellow No. 6 (UNII: H77VEI93A8) Fumaric Acid (UNII: 88XHZ13131) Hypromelloses (UNII: 3NXW29V3WO) Magnesium Stearate (UNII: 70097M6I30) Mannitol (UNII: 3OWL53L36A) Maltodextrin (UNII: 7CVR7L4A2D) Cellulose, Microcrystalline (UNII: OP1R32D61U) Talc (UNII: 7SEV7J4R1U) Product Characteristics Color ORANGE Score no score Shape ROUND Size 8mm Flavor ORANGE Imprint Code C285 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59746-285-32 3 in 1 CARTON 02/19/2015 1 30 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091116 02/19/2015 CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59746-286 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Cetirizine Hydrochloride (UNII: 64O047KTOA) (Cetirizine - UNII:YO7261ME24) Cetirizine Hydrochloride 10 mg Inactive Ingredients Ingredient Name Strength Acesulfame Potassium (UNII: 23OV73Q5G9) Aspartame (UNII: Z0H242BBR1) Betadex (UNII: JV039JZZ3A) Silicon Dioxide (UNII: ETJ7Z6XBU4) Croscarmellose Sodium (UNII: M28OL1HH48) .alpha.-tocopherol, Dl- (UNII: 7QWA1RIO01) Ethylcelluloses (UNII: 7Z8S9VYZ4B) Fd&c Yellow No. 6 (UNII: H77VEI93A8) Fumaric Acid (UNII: 88XHZ13131) Hypromelloses (UNII: 3NXW29V3WO) Magnesium Stearate (UNII: 70097M6I30) Mannitol (UNII: 3OWL53L36A) Maltodextrin (UNII: 7CVR7L4A2D) Cellulose, Microcrystalline (UNII: OP1R32D61U) Talc (UNII: 7SEV7J4R1U) Product Characteristics Color ORANGE Score no score Shape ROUND Size 11mm Flavor ORANGE Imprint Code C286 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59746-286-32 3 in 1 CARTON 02/19/2015 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091116 02/19/2015 Labeler - Jubilant Cadista Pharmaceuticals Inc. (022490515) Registrant - Jubilant Generics Limited (650801538) Establishment Name Address ID/FEI Business Operations Jubilant Generics Limited Roorkee 650369221 MANUFACTURE(59746-285, 59746-286)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.