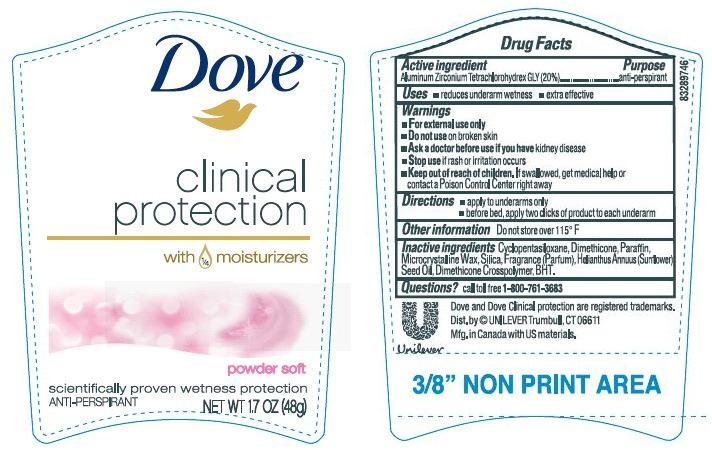
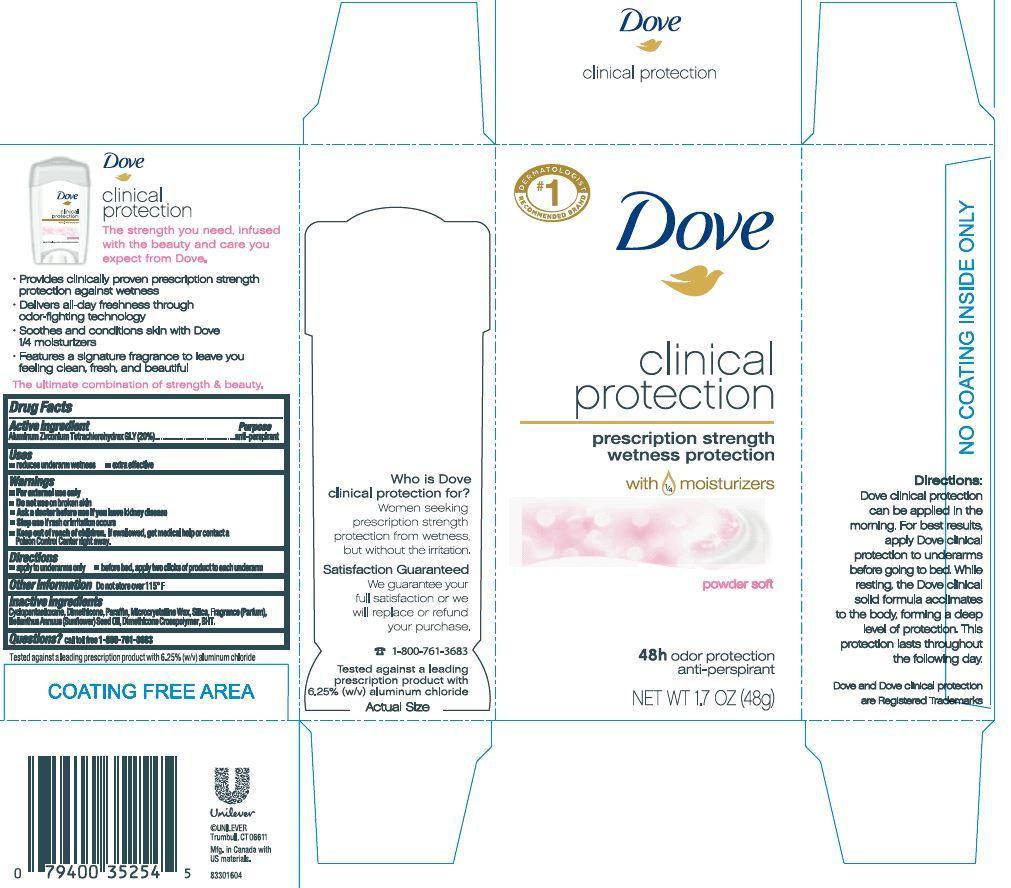
DOVE CLINICAL PROTECTION POWDER SOFT ANTIPERSPIRANT AND DEODORANT- aluminum zirconium tetrachlorohydrex gly stick
Dove by
Drug Labeling and Warnings
Dove by is a Otc medication manufactured, distributed, or labeled by Conopco Inc. d/b/a Unilever. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- QUESTIONS
- 1.7 oz PDP
- 1.7 oz carton
-
INGREDIENTS AND APPEARANCE
DOVE CLINICAL PROTECTION POWDER SOFT ANTIPERSPIRANT AND DEODORANT
aluminum zirconium tetrachlorohydrex gly stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 64942-1393 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Aluminum Zirconium Tetrachlorohydrex GLY (UNII: 8O386558JE) (Aluminum Zirconium Tetrachlorohydrex GLY - UNII:8O386558JE) Aluminum Zirconium Tetrachlorohydrex GLY 20 g in 100 g Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SUNFLOWER OIL (UNII: 3W1JG795YI) PARAFFIN (UNII: I9O0E3H2ZE) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64942-1393-2 1 in 1 CARTON 1 NDC: 64942-1393-1 48 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 12/15/2015 Labeler - Conopco Inc. d/b/a Unilever (001375088)
Trademark Results [Dove]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DOVE 98494611 not registered Live/Pending |
Mars, Incorporated 2024-04-11 |
 DOVE 98456408 not registered Live/Pending |
Wine Country Management Inc. 2024-03-19 |
 DOVE 97764111 not registered Live/Pending |
MARS, INCORPORATED 2023-01-23 |
 DOVE 97655383 not registered Live/Pending |
Conopco, Inc. 2022-10-31 |
 DOVE 97616732 not registered Live/Pending |
MARS, INCORPORATED 2022-10-03 |
 DOVE 97407947 not registered Live/Pending |
Conopco, Inc. 2022-05-12 |
 DOVE 90576914 not registered Live/Pending |
Planet Labs Inc. 2021-03-12 |
 DOVE 90232806 not registered Live/Pending |
Conopco, Inc. 2020-10-02 |
 DOVE 87604241 5782412 Live/Registered |
Mars, Incorporated 2017-09-12 |
 DOVE 86354716 4820803 Live/Registered |
Dove Surfing Wetsuits Limited Company 2014-08-01 |
 DOVE 86280653 4687543 Live/Registered |
Stoma Ventures, LLC 2014-05-14 |
 DOVE 86200365 4620308 Live/Registered |
Mars, Incorporated 2014-02-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.