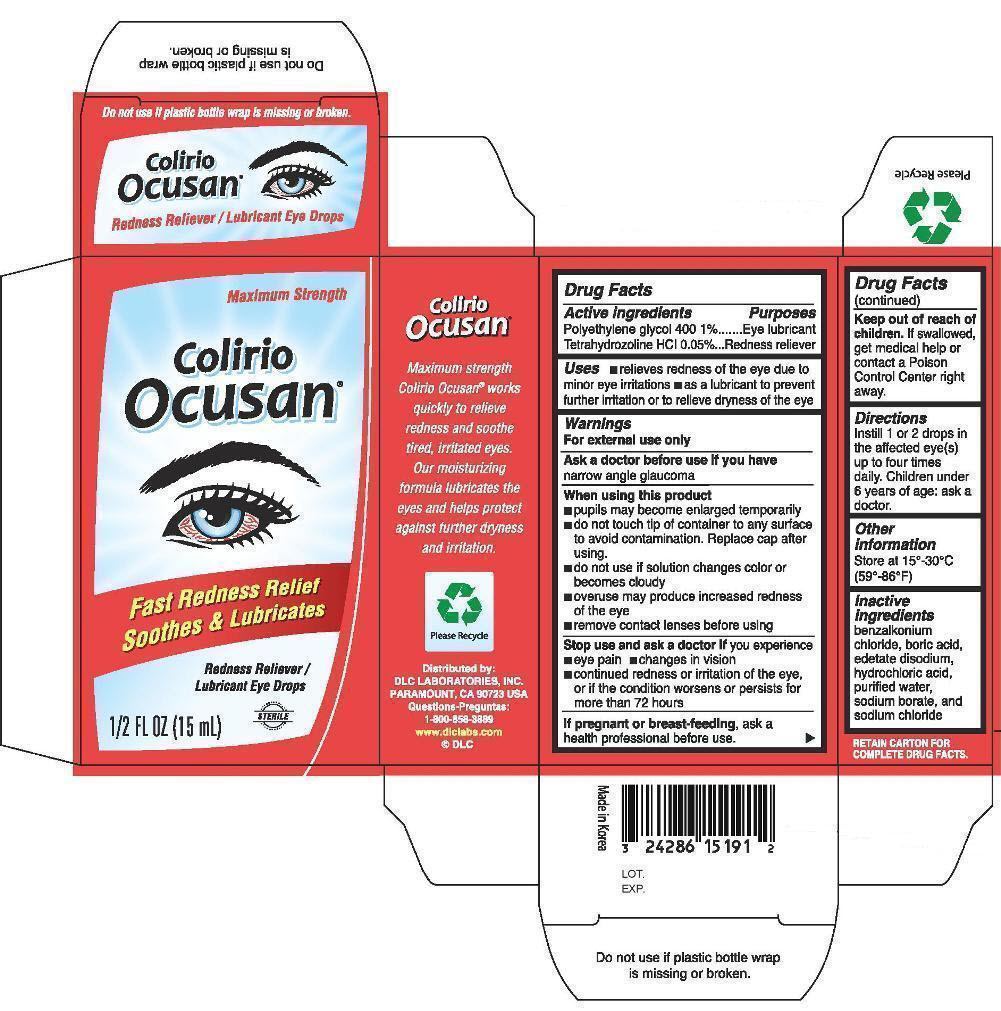
Colirio Ocusan by DLC Laboratories, Inc. Drug Facts
Colirio Ocusan by
Drug Labeling and Warnings
Colirio Ocusan by is a Otc medication manufactured, distributed, or labeled by DLC Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
COLIRIO OCUSAN- polyethlene glycol 400 tetrahydrozoline hcl liquid
DLC Laboratories, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
- relieves redness of the eye due to minor eye irritations
- as a lubricant to prevent further irritation or to relieve dryness of the eye
Warnings
For external use only
When using this product
- pupils may become enlarged temporarily
- do not touch tip of container to any surface to avoid contamination. Replace cap after using.
- do not use if solution changes color or becomes cloudy
- overuse may produce increased redness of the eye
- remove contact lenses before using
Stop use and ask a doctor if
you experience
- eye pain
- changes in vision
- continued redness or irritation of the eye, or if the condition worsens or persists for more than 72 hours
Directions
Instill 1 or 2 drops in the affected eye(s) up to four times daily. Children under 6 years of age: ask a doctor.
| COLIRIO OCUSAN
polyethlene glycol 400 tetrahydrozoline hcl liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - DLC Laboratories, Inc. (093351930) |
Revised: 1/2020
Document Id: 63ac1e24-fb60-451e-9711-b0ecc175556f
Set id: 3bf7882e-cd47-432f-9eaf-ea1a6cdeeb26
Version: 7
Effective Time: 20200106
DLC Laboratories, Inc.