CERETEC- technetium tc-99m exametazime injection, powder, lyophilized, for solution
CERETEC by
Drug Labeling and Warnings
CERETEC by is a Prescription medication manufactured, distributed, or labeled by Medi-Physics Inc. dba GE Healthcare., GE Healthcare AS. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
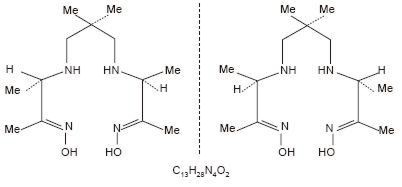
The Ceretec kit is supplied as a pack of 5 vials for use in the preparation of a technetium Tc99m exametazime intravenous injection as a diagnostic radiopharmaceutical for use as an adjunct in the detection of altered regional cerebral perfusion and for the radiolabeling of autologous leukocytes. Each vial of Ceretec contains a pre-dispensed sterile, non-pyrogenic, lyophilized mixture of 0.5 mg exametazime [(RR,SS)-4.8-diaza-3,6,6,9-tetramethylundecane-2, 10-dione bisoxime], 7.6 µg stannous chloride dihydrate (minimum stannous tin 0.6 µg; maximum total stannous and stannic tin 4.0 µg per vial) and 4.5 mg sodium chloride, sealed under nitrogen atmosphere with a rubber closure. The product contains no antimicrobial preservative.
Prior to publication of the USAN, exametazime was formerly known as hexamethylpropylene amine oxime (HM-PAO). The name HM-PAO appears in many publications.
The structural formula of exametazime is:
When sterile pyrogen-free sodium pertechnetate Tc99m in isotonic saline is added to the vial of Ceretec, a Tc99m complex of exametazime is formed.
Administration is by intravenous injection for diagnostic use.
Physical Characteristics
Technetium Tc99m decays by isomeric transition with a physical half-life of 6.03 hours.(1) Photons that are useful for imaging studies are listed in Table 1.
Table 1. Principal Radiation Emission Data-technetium Tc99m Radiation Mean %/
DisintegrationMean Energy
(keV)(1) Dillman, L.T. and Von der Lage, F.C. Radionuclide decay schemes and nuclear parameters for use in radiation-dose estimation. MIRD Phamphlet No. 10, p. 62, 1975. Gamma 2 87.87 140.5 External Radiation
The specific gamma ray constant for technetium Tc99m is 206 microCoulomb kg-1/37 MBq-h, (0.8 R/millicurie-h) at 1 cm. The first half-value thickness of lead (Pb) for technetium Tc99m is 0.2 mm. A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of Pb is shown in Table 2. For example, the use of a 2.7 mm thickness of Pb will decrease the external radiation exposure by a factor of 1,000.
Table 2. Radiation Attenuation by Lead Shielding Shield Thickness
(Pb) mmCoefficient of Attenuation 0.2 0.5 0.95 10–1 1.8 10–2 2.7 10–3 3.6 10–4 4.5 10–5 To correct for physical decay of this radionuclide, the fractions that remain at selected intervals relative to the time of calibration are shown in Table 3.
-
CLINICAL PHARMACOLOGY
General
When technetium Tc99m pertechnetate is added to exametazime in the presence of stannous reductant, a lipophilic technetium Tc99m complex is formed. This lipophilic complex is the active moiety. It converts at approximately 12%/hour to less lipophilic species. When the secondary complex is separated from the lipophilic species, it is unable to cross the blood-brain-barrier. The useful life of the reconstituted agent is limited to 30 minutes.
Pharmacokinetics
Studies in normal volunteers have shown that the technetium Tc99m complex of the RR,SS(d,l) diastereoisomer of exametazime is rapidly cleared from the blood after intravenous injection. Uptake in the brain reaches a maximum of 3.5-7.0% of the injected dose within one minute of injection. Up to 15% of the activity is eliminated from the brain by 2 minutes post injection, after which little activity is lost for the following 24 hours except by physical decay of technetium Tc99m. The activity not associated with the brain is widely distributed throughout the body, particularly in muscle and soft tissue. About 30% of the injected dose is found in the gastrointestinal tract immediately after injection and about 50% of this is excreted through the intestinal tract over 48 hours. Also, about 40% of the injected dose is excreted through the kidneys and urine over the 48 hours after injection.
Leukocyte
The lipophilic Tc99m exametazime complex is taken up by leukocytes, and selectively retained in neutrophils. Label elution rate is up to 10% in the first hour.
Pharmacodynamics
Tc99m-labeled leukocyte: Based upon in vivo recovery and very low lung and liver uptake, the labeled leukocytes are still functional. Following reinjection of the Tc99m labeled leukocytes the circulating granulocyte activity as a percentage of labeled granulocyte activity at 40 minutes after injection gave a mean of 37% (range 10-47%).
During the first hour following injection of Tc99m labeled leukocytes, activity is seen in the lungs, liver, spleen, blood pool, bone marrow and the bladder. The kidneys (parenchyma and/or renal pelvis) and gall bladder may also be visualized. Over the first 1-6 hours, the Tc99m is visualized in the bowel. At 24 hours post-injection substantial colonic activity is seen. The normal areas visualized in earlier scans are still visible.
-
CLINICAL TRIALS
Two clinical trials were performed in a total of 88 patients who had suspected intra-abdominal infection or inflammation. Subjects received both Tc99m labeled leukocytes and In-111 labeled leukocytes. Images were obtained at 2 and 30 minutes and at 2 and 4 hours and 24 hours. In two other clinical trials, in a total of 127 patients with suspected abdominal inflammation or infection received Tc99m labeled leukocytes. Imaging was at 24 hours in one study and at 1, 3 and 24 hours in the other. In all 4 studies, images were blindly evaluated and the findings were confirmed by surgery, biopsy or other clinical data.
Based on the above 4 studies, between 2 to 4 hours Tc99m labeled leukocytes had 95-100% sensitivity and 62-85% specificity with similar numbers of false positive and false negative findings. The value of the 24 hour Tc99m labeled leukocyte images is inconsistent. In all studies the false positive and false negatives relate to the bowel background, the location of the site of infection/inflammation and whether or not it is contiguous with the bowel. The 24 hour films should be interpreted with great caution because of a high bowel background; false negatives were noted in both Tc99m and In-111 labeled leukocytes.
Other studies suggest that the interpretation of the images could be affected by the presence of tumors, infarction and peritonitis, etc. Liver abscess may be missed because of the bowel background. Caution should be exercised in making the final diagnosis.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
As with any injected product, acute hypersensitivity or allergic reactions are possible. Limited reports have been received of hypersensitivity reactions following administration of Tc99m labeled leukocytes prepared using Tc99m exametazime. However, the materials used in leukocyte cell separation may cause hypersensitivity reactions. It is essential that cells are washed free of sedimentation agents before they are reinjected into the patient.
In case of side effects following administration of radiopharmaceuticals, users should ensure the availability of appropriate medical treatment at the time of administration of any radiopharmaceutical to the patient.
A thorough knowledge of the normal distribution of intravenously administered technetium Tc99m exametazime injection is essential in order to interpret pathologic studies accurately. Caution should be exercised in making the final diagnosis. Results can be affected by the presence of tumor, infarction, peritonitis, non-gastrointestinal or bony sites of inflammatory cell collections.
The contents of the Ceretec vial are not radioactive. After the sodium pertechnetate Tc99m is added, the product is radioactive and adequate shielding of the final preparation must be maintained. The contents of the Ceretec vial are intended only for use in preparation of technetium Tc99m exametazime injection and are NOT to be administered directly to the patient.
General
The contents of the Ceretec vial are sterile and pyrogen free. The vial contains no bacteriostatic preservative. It is essential that the user follow the directions carefully and adhere to strict aseptic procedures during preparation of the radiopharmaceutical.
Radiopharmaceuticals should be used only by or under the control of physicians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
To minimize radiation dose to the bladder, the patient should be encouraged to void when the examination is completed and as often thereafter as possible. Adequate hydration should be encouraged to permit frequent voiding.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term animal studies have not been performed to evaluate carcinogenic potential or whether exametazime affects fertility in males or females. When evaluated in the Ames test, exametazime increased the apparent rate of gene mutation in the TA100 strain of S. typhimurium. Exametazime did not cause chromosomal aberrations in vitro (Chinese Hamster Ovary cells) or in vivo (rat bone marrow).
Pregnancy Category C
Animal reproduction studies have not been conducted with Tc99m exametazime. It is also not known whether Tc99m exametazime can cause fetal harm when administered to a pregnant woman or if it can affect reproductive capacity. Therefore, Tc99m exametazime should not be administered to a pregnant woman unless the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Technetium Tc99m is excreted in human milk during lactation. It is not known whether exametazime is excreted in human milk. Therefore, formula feedings should be substituted for breast feeding for 60 hours.
Geriatric Use
Clinical studies of Ceretec™ did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
Tc99m labeled leukocytes for adjunctive localization of intra-abdominal infection or inflammation.
The normal adult (70 kg) dose is 0.259-0.925 GBq (7-25 mCi) as Tc99m labeled leukocytes by intravenous injection. Optimal planar imaging is between 2-4 hours.
Cerebral Scintigraphy
The recommended dose range for i.v. administration, of reconstituted sodium pertechnetate Tc99m exametazime in the average adult (70 kg) is 370-740 MBq (10-20 mCi).
Dynamic imaging may be performed between 0 to 10 minutes following injection. Static imaging may be performed from 15 minutes up to 6 hours after injection.
-
INSTRUCTIONS FOR PREPARATION AND USE
GENERAL PREPARATION PRECAUTIONS
The technetium Tc99m labeling reaction involved in preparing technetium Tc99m exametazime injection depends on maintaining tin in the divalent (reduced) state. Any oxidant present in the sodium pertechnetate Tc99m employed may adversely affect the quality of the preparation. Sodium pertechnetate Tc99m containing oxidants should not be used for the preparation of the labeled product. To meet the last requirement, a generator must be eluted within 24 hours prior to obtaining any elute for reconstitution with the Ceretec kit.
Sodium Chloride Injection, USP must be used as the diluent. Do not use bacteriostatic sodium chloride as a diluent for sodium pertechnetate Tc99m injection because it will increase the oxidation products and adversely affect the biological distribution of Ceretec.
The contents of the Ceretec vial are sterile and pyrogen free. The vial contains no bacteriostatic preservative. It is essential that the user follow the directions carefully and adhere to strict aseptic procedures during preparation of the radiopharmaceutical.
Technetium Tc99m exametazime injection, like other radioactive drugs, must be handled with care and appropriate safety measures should be used to minimize radiation exposure to clinical personnel. Care should also be taken to minimize radiation exposure to the patient consistent with proper patient management.
Care should be taken when handling blood specimens to be labeled using this radiopharmaceutical. Even if the subject has been tested, no method can offer complete assurance that Hepatitis B Virus, Human Immuno-deficiency Virus (HIV) or other infectious agents are absent. All human blood samples should be considered potentially infectious. Precautions for handling are as those for handling radioactive materials.
Procedure for the Preparation of Technetium Tc99m Exametazime Injection
Note: Sterile technique must be used throughout. The user should wear waterproof gloves during the handling and administration procedure.
- 1) Place one of the vials in a suitable shielding container and sanitize the rubber septum with an isopropyl alcohol swab.
- 2) Using a 10 mL syringe, inject into the shielded vial 5 mL of sterile eluate from a technetium Tc99m generator (see cautionary notes 1-3). Before withdrawing the syringe from the vial, withdraw 5 mL of gas from the space above the solution to normalize the pressure in the vial. Gently invert the shielded vial for 10 seconds to ensure complete dissolution of the powder.
- 3) Assay the total activity and calculate the volume to be injected. The patient dose should be measured in a suitable radioactivity calibration system immediately prior to administration.
- 4) Complete the label provided and attach to the vial shield. The technetium Tc99m exametazime injection is ready for quality control.
- 5) Maintain adequate shielding of the radioactive preparation.
- 6) Do not use the preparation more than 30 minutes after time of formulation. Discard any unused material.
- 7) Visually inspect the reconstituted material at a safe distance behind lead glass, and do not use if there is evidence of foreign matter.
- 8) The injection may be prepared for use in cerebral scintigraphy or for use in the preparation of Tc99m labeled white blood cells (WBCs).
- 9) The pH of the prepared injection is 9.0-9.8.
- 10) Also see section on Cautionary Notes for all Preparations.
Procedure for Radiolabeling of Autologous Leukocytes with Technetium Tc99m Exametazime Injection
Note: Sterile technique must be used throughout. The user should wear waterproof gloves during the handling and administration procedure.
- 1) Prepare a 60 mL syringe containing 10 mL acid citrate dextrose solution.
- 2) Using aseptic venipuncture technique and the prepared syringe (from Step 1) fitted with a 19 or 20 gauge needle, withdraw approximately 40 mL whole blood from the patient. (Blood withdrawal should be smooth and slow so as not to produce bubbles or foaming). Cap syringe after withdrawing blood.
- 3) Gently mix the contents of the syringe.
- 4) Clamp the syringe barrel to the ring stand in an upright (needle side up) position and tilt the syringe 10-20 degrees from its position perpendicular to the bench.
- 5) Allow the red cells to sediment 30-60 minutes, depending upon when the supernatant [leukocyte rich plasma (LRP)] looks clear of red blood cells.
- 6) Replace the capped needle with a winged infusion set.
- 7) Collect the plasma LRP into a centrifuge tube marked WBC by expressing the LRP through the infusion set tubing assuring the red cells do not enter the container.
- 8) Immediately centrifuge the capped WBC tube at 400-450 g for 5 minutes.
- 9)
Transfer the supernatant to the leukocyte poor plasma (LPP) tube allowing enough supernatant to cover the white cell button.
(Note: the button often contains a small number of red cells and may appear red.) Reserve LPP for later use (steps 12, 16, 19). - 10) Wash the white cell button with approximately 5.0 mL Sodium Chloride Injection, USP (0.9%). Cap the tube and resuspend the button by gently swirling.
- 11) Centrifuge the capped WBC tube at 150 g for 8 minutes and discard all but 0.5-1.0 mL of the supernate to cover the cells.
- 12) Add 1.0 mL of LPP to the white cell button and resuspend the cells by gentle swirling.
- 13) Reconstitute a vial of Ceretec with approximately 30 mCi of Tc99m pertechnetate in 5.0 mL Sodium Chloride (0.9%) Injection, according to the procedure outlined below. Generator eluate more than 2 hours old should not be used. Parenteral drug products should be inspected visually for particulate matter and discoloration before administration.
- 14) Add the Tc99m Ceretec to the WBC tube. Swirl gently to mix.
- 15) Set a lab timer for 15 minutes and allow the white cells to incubate. Swirl periodically during the incubation.
- 16) After incubation, withdraw about 10 mL of the LPP and add to the white cell suspension in the WBC tube.
- 17) Cap the WBC tube, gently swirl, and then centrifuge at 450 g for 5 minutes.
- 18) Decant the supernatant in the WBC tube into the wash tube and leave the labeled white cells in the WBC tube.
- 19) Add approximately 5 mL of LPP to the WBC tube. Resuspend the cells by gentle swirling.
- 20) When the cells are in suspension, withdraw the cells into a syringe. Cap the syringe and assay the amount of radioactivity in a dose calibrator.
- 21) Place the syringe in a lead shielded container.
- 22) Administer the Tc99m labeled leukocyte suspension using a 19G needle as soon as possible after labeling.
- 23) Also see section of Cautionary Notes for all Preparations.
Cautionary Notes for all Preparations
- 1) 0.37 GBq up to 2.00 GBq (10 mCi up to 54 mCi) technetium Tc99m may be added to the vial. Before reconstitution the technetium Tc99m generator eluate may be adjusted to the correct radioactive concentration to a volume of 5 mL by dilution with preservative-free, non-bacteriostatic saline for injection.
- 2) Use only eluate from a technetium Tc99m generator which was previously eluted within 24 hours. Generator eluate more than 2 hours old should not be used. For the highest radiochemical purity reconstitute with freshly eluted technetium Tc99m generator eluate. When reconstituting a vial of Ceretec with 31 to 54 mCi, generator eluate more than 30 minutes old should not be used.
- 3) Radiochemical purity testing must be performed prior to patient administration. A radiochemical purity greater than 80% is necessary for product acceptance.
- 4) Do not use the final radiopharmaceutical preparation more than 30 minutes after the time of reconstitution. Discard any unused material.
Quality Control
Radiochemical purity determination must be performed before administration to the patient. Three potential radiochemical impurities may be present in the prepared injection of the lipophilic technetium Tc99m exametazime complex.
These are a secondary technetium Tc99m exametazime complex, free pertechnetate, and reduced-hydrolyzed-technetium Tc99m. A combination of 3 chromatographic systems is necessary for the complete definition of the radiochemical composition of the injection.
The following protocol has been designed to enable analysis of the radiochemical purity of Ceretec (99mTc-exametazime). It should be started within 2 minutes of reconstitution. The entire procedure takes approximately 15 minutes.
Equipment and Eluents
- 1) Quality control kit which includes all necessary components
- 2)
Individual supplies:
SA ITLC strips 20 cm × 2.0 cm
Whatman No. 1 strips 6 cm × 0.7 cm
MEK (methyl ethyl ketone [butanone]) (99.9 + % HPLC Grade)
0.9% aqueous sodium chloride (non-bacteriostatic)
50% aqueous acetonitrile (99.9 + % HPLC Grade)
Dilute with non-bacteriostatic Water for Injection
Glass test tubes (12 × 75 mm)
Glass measuring cylinders (100ml) with covers
1 mL syringes with 25 gauge needles - 3) Suitable counting equipment.
Method
- 1) Prepare one chromatography tube containing 0.2-0.3 mL of 50% acetonitrile. Prepare two 100 ml cylinders each containing a 1 cm depth of fresh MEK and 0.9% sodium chloride, respectively. Identify the solvent in each cylinder.
- 2) Prepare two SA ITLC strips and one Whatman No. 1 paper strip. Mark the Whatman strip 1.0 cm from the bottom as the point of origin. Mark the SA ITLC strips 2.5cm from the bottom as the point of origin. Mark both the SA ITLC strips at 14 cm above the origin (solvent front).
- 3) Reconstitute a Ceretec vial according to this insert.
- 4) Apply at least 5 µL samples of Ceretec to the origin of the 3 strips within 15 minutes of reconstitution. Immediately place one SA ITLC strip into the MEK tank, the second SA ITLC strip into the saline tank and the Whatman No. 1 paper strip into the 50% acetonitrile tube. Make sure strips are not adhering to the sides of the container.
- 5) The SA ITLC MEK strip takes approximately 15 minutes to run. When the eluate has reached the solvent front mark remove the strip from the tube with forceps and immediately cut 1.0 cm above the origin.
- 6) The SA ITLC saline strip takes approximately 15 minutes to run. When the eluate has reached the solvent front mark remove the strip from the tube with forceps and immediately cut 2.5 cm above the origin.
- 7) The Whatman No. 1 paper CH3CN strip takes approximately 100 seconds to run. When the eluate has reached the solvent front remove the strip from the tube with forceps and immediately cut 0.5 cm above the origin.
- 8) Count the separate sections of each strip to determine the activity distribution. Make sure proper counting geometry is maintained attempting to reduce any interference from equipment dead time.
- 9)
Determine:
% bottom of saline strip – % bottom of MEK strip
(= % lipophilic exametazime complex)
% top of saline strip (= % pertechnetate)
% bottom of Whatman No. 1 paper strip (= % reduced-hydrolyzed-Tc)
A radiochemical purity of >80% may be expected provided the measurement has been carried out within 4 hours of reconstitution for stabilized Ceretec and 30 minutes for Ceretec used for WBC labeling.
Interpretation of Chromatogram
System 1 (SA ITLC: MEK [butanone])
Secondary Tc exametazime complex and reduced-hydrolyzed-Tc remain at the origin.
Lipophilic Tc exametazime complex and pertechnetate migrate at Rf 0.8-1.0.
-
RADIATION DOSIMETRY
Based on human data, the absorbed radiation doses to an average human adult (70 kg) from an intravenous injection of this product are estimated below. The values are listed as µGy/MBq, rads/mCi with urination every 2 hours. Bladder wall dose is 19 µGy/MBq, 0.07 rads/mCi with 4 hour urination and 89 µGy/MBq, 0.33 rads/mCi with no urination.
Table 4. Estimated Absorbed Radiation Dose* for Cerebral Scintigraphy Target Organ Absorbed radiation dose Tc99m exametazime injection µGy/MBq rads/mCi mGy/740 MBq rads/20 mCi - * Data supplied by Oak Ridge Associated Universities, Radiopharmaceutical Internal Dose Information Center.
Lachrymal Glands 69.4 0.258 51.36 5.16 Gallbladder Wall 51.0 0.19 37.74 3.80 Kidney 35.0 0.13 25.90 2.60 Thyroid 27.0 0.10 19.98 2.00 Upper Large 21.0 0.079 15.54 1.58 Intestine Wall Liver 15.0 0.054 11.10 1.08 Small Intestine Wall 12.0 0.044 8.88 0.88 Lower Large 15.0 0.054 11.10 1.08 Intestine Wall Urinary Bladder Wall 13.0 0.047 9.62 0.94 Brain 6.9 0.026 5.11 0.52 Ovaries 6.3 0.023 4.66 0.46 Testes 1.8 0.007 1.33 0.14 Whole Body 3.6 0.013 2.66 0.26 Red Marrow 3.4 0.013 2.52 0.26 Bone Surfaces 4.8 0.018 3.55 0.36 Eyes 6.9 0.026 5.11 0.52 Table 5. In vivo Localization of Tc99m Labeled Leukocytes The estimated absorbed radiation doses to various organs following the intravenous administration of Tc99m labeled leukocytes given by ICRP 53* are as follows (bladder voiding every 3.5 hours) Target Organ Absorbed Radiation Dose (mGy per 200 MBq) rads/25 mCi Effective Dose Equivalent (EDE) 3.4 mSv/200 MBq. - * International Commission on Radiological Protection, "Radiation Dose to Patients from Radiopharmaceuticals", ICRP 53, 1988.
Spleen 30 13.89 Red Marrow 4.4 2.04 Liver 4 1.85 Pancreas 2.8 1.3 Ovaries 0.84 0.39 Testes 0.34 0.16 Uterus 0.76 0.35 -
HOW SUPPLIED
The kit comprises 5 individual vials of sterile, non-pyrogenic, freeze-dried mixture of exametazime, stannous chloride dihydrate and sodium chloride, 5 radiation labels, 5 radiochemical purity worksheets, 5 labeling efficiency worksheets, 1 leukocyte labeling schematic and 1 package insert.
NDC: 17156-022-05
Storage
Store the kit at 15°-25°C (59°-77°F).
Store the formulated drug for up to 30 minutes at 20°-25°C (68°-77°F) using appropriate radiation shielding.
This reagent kit is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant to 32 Ill. Code Adm. Section, Section 330.260(a) and 335.4010 or under equivalent licenses of the U.S. Nuclear Regulatory Commission, or an Agreement State.
-
SPL UNCLASSIFIED SECTION
Patent No. 4,789,736
Distributed by:
GE Healthcare
Medi-Physics, Inc.
Arlington Heights, IL 60004Customer Service:
1-800-292-8514
Professional Services:
1-800-654-0118Manufactured by:
GE Healthcare AS
Oslo, NorwayCeretec is a trademark of GE Healthcare.
GE and the GE Monogram are trademarks of General Electric Company.
© 2013 General Electric Company – All rights reserved.
43P-9159F-OSLO
Revised March 2013
-
PRINCIPAL DISPLAY PANEL - 5 Vial Kit
GE Healthcare
CERETEC™
Technetium Tc99m Exametazime InjectionCode N109
5-vial kitNDC: 17156-022-05
Storage: Store the kit at 15°-25°C (59°-77°F).
After reconstitution with Technetium Tc99m,
store at 20°-25°C (68°-77°F).Use appropriate radiation shielding.
Not for use in humans until technetium
Tc99m is added.For intravenous use as directed.
For Dosage and Administration:
See Package Insert.Content. Each package contains the following:
Five Ceretec vials. Each Ceretec vial contains a
lyophilized form of 0.5 mg exametazime. 7.6 μg,
stannous chloride dihydrate (minimum stannous
tin 0.6 μg, maximum total stannous and stannic
tin 4.0 μg per vial) and 4.5 mg sodium chloride.For preparation of Technetium Tc99m
Exametazime Injection see package insert.44P-9159F-OSLO
-
INGREDIENTS AND APPEARANCE
CERETEC
technetium tc-99m exametazime injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 17156-022 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TECHNETIUM TC-99M EXAMETAZIME (UNII: 3B744AG22N) (TECHNETIUM TC-99M EXAMETAZIME - UNII:3B744AG22N) EXAMETAZIME 0.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength Stannous Chloride (UNII: 1BQV3749L5) 7.6 ug in 5 mL Sodium Chloride (UNII: 451W47IQ8X) 4.0 mg in 5 mL Nitrogen (UNII: N762921K75) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17156-022-05 5 in 1 TRAY 12/30/1988 1 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019829 12/30/1988 Labeler - Medi-Physics Inc. dba GE Healthcare. (095263729) Establishment Name Address ID/FEI Business Operations GE Healthcare AS 515048908 MANUFACTURE(17156-022) , RELABEL(17156-022) , REPACK(17156-022) , API MANUFACTURE(17156-022)
Trademark Results [CERETEC]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CERETEC 73715173 1645766 Live/Registered |
AMERSHAM INTERNATIONAL PLC 1988-03-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.