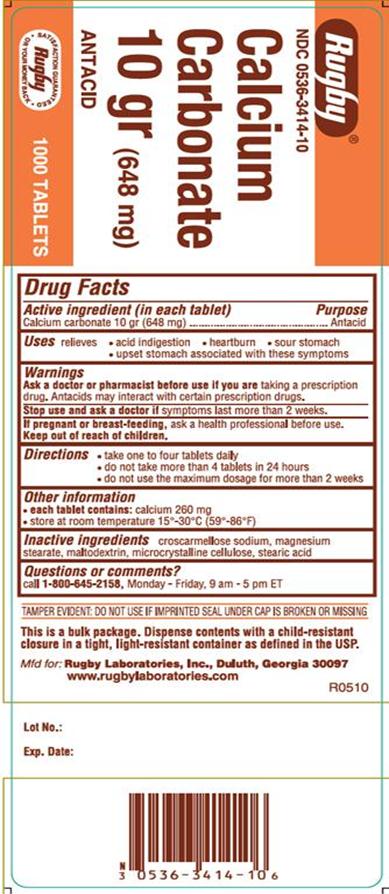
Calcium Carbonate Tablets
Calcium carbonate by
Drug Labeling and Warnings
Calcium carbonate by is a Otc medication manufactured, distributed, or labeled by Rugby Laboratories Inc., Advance Pharmaceutical Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CALCIUM CARBONATE- calcium carbonate tablet
Rugby Laboratories Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Calcium Carbonate Tablets
Warnings
Ask a doctor or pharmacist before use if you are taking a prescription drug. Antacids may interact with certain prescription drugs.
Stop use and ask a doctor if symptoms last more than 2 weeks.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
Directions
- take one to four tablets daily
- do not take more than 4 tablets in 24 hours
- do not use the maximum dosage for more than 2 weeks
Other Information
- Each tablet contains: calcium 260 mg
- store at room temperature 15°-30°C (59°-86°F)
Inactive Ingredients
croscarmellose sodium, magnesium sterate, maltodextrin, microcrystalline cellulose, stearic acid
Questions or Comments
Call 1-800-645-2158, Monday – Friday, 9 am – 5 pm ET
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SEAL UNDER CAP IS BROKEN OR MISSING
This is a bulk pakage, dispense contents with a child-resistant closure in a tight, light-resistant container as defined in the USP.
Mfd for: Rugby Laboratories,Inc
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 0536-3414-10
Rugby
Calcium Carbonate
Antacid
1000 Tablets
| CALCIUM CARBONATE
calcium carbonate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Rugby Laboratories Inc. (079246066) |