METHADONE HYDROCHLORIDE tablet
METHADONE HYDROCHLORIDE by
Drug Labeling and Warnings
METHADONE HYDROCHLORIDE by is a Prescription medication manufactured, distributed, or labeled by H.J. Harkins Company Inc., Mallinckrodt Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
BOXED WARNING
(What is this?)
Deaths, cardiac and respiratory, have been reported during initiation and conversion of pain patients to methadone treatment from treatment with other opioid agonists. It is critical to understand the pharmacokinetics of methadone when converting patients from other opioids (see DOSAGE AND ADMINISTRATION). Particular vigilance is necessary during treatment initiation, during conversion from one opioid to another, and during dose titration.
Respiratory depression is the chief hazard associated with methadone hydrochloride administration. Methadone's peak respiratory depressant effects typically occur later, and persist longer than its peak analgesic effects, particularly in the early dosing period. These characteristics can contribute to cases of iatrogenic overdose, particularly during treatment initiation and dose titration.
In addition, cases of QT interval prolongation and serious arrhythmia (torsades de pointes) have been observed during treatment with methadone. Most cases involve patients being treated for pain with large, multiple daily doses of methadone, although cases have been reported in patients receiving doses commonly used for maintenance treatment of opioid addiction.
Methadone treatment for analgesic therapy in patients with acute or chronic pain should only be initiated if the potential analgesic or palliative care benefit of treatment with methadone is considered and outweighs the risks.
Conditions for Distribution and Use of Methadone Products for the Treatment of Opioid Addiction
Code of Federal Regulations, Title 42, Sec 8
Methadone products when used for the treatment of opioid addiction in detoxification or maintenance programs, shall be dispensed only by opioid treatment programs (and agencies, practitioners or institutions by formal agreement with the program sponsor) certified by the Substance Abuse and Mental Health Services Administration and approved by the designated state authority. Certified treatment programs shall dispense and use methadone in oral form only and according to the treatment requirements stipulated in the Federal Opioid Treatment Standards (42 CFR 8.12). See below for important regulatory exceptions to the general requirement for certification to provide opioid agonist treatment.
Failure to abide by the requirements in these regulations may result in criminal prosecution, seizure of the drug supply, revocation of the program approval, and injunction precluding operation of the program.
-
SPL UNCLASSIFIED SECTION
Regulatory Exceptions To The General Requirement For Certification To Provide Opioid Agonist Treatment:
- During inpatient care, when the patient was admitted for any condition other than concurrent opioid addiction (pursuant to 21 CFR 1306.07(c)), to facilitate the treatment of the primary admitting diagnosis.
- During an emergency period of no longer than 3 days while definitive care for the addiction is being sought in an appropriately licensed facility (pursuant to 21 CFR 1306.07(b)).
-
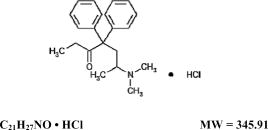
DESCRIPTION
Methadone Hydrochloride Tablets USP for oral administration, each contain 5 mg or 10 mg of methadone hydrochloride.
Methadone hydrochloride is a white, crystalline material that is water-soluble.
Methadone hydrochloride is chemically described as 6-(dimethylamino)-4,4-diphenyl-3-heptanone hydrochloride. Methadone hydrochloride has a melting point of 235°C, and a pKa of 8.25 in water at 20°C. Its octanol/water partition coefficient at pH 7.4 is 117. A solution (1:100) in water has a pH between 4.5 and 6.5.
It has the following structural formula:
The tablets also contain lactose monohydrate, magnesium stearate, microcrystalline cellulose and silicon dioxide.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Methadone hydrochloride is a mu-agonist; a synthetic opioid analgesic with multiple actions qualitatively similar to those of morphine, the most prominent of which involves the central nervous system and organs composed of smooth muscle. The principal therapeutic uses for methadone are for analgesia and for detoxification or maintenance in opioid addiction. The methadone abstinence syndrome, although qualitatively similar to that of morphine, differs in that the onset is slower, the course is more prolonged, and the symptoms are less severe.
Some data also indicate that methadone acts as an antagonist at the N-methyl-D-aspartate (NMDA) receptor. The contribution of NMDA receptor antagonism to methadone's efficacy is unknown. Other NMDA receptor antagonists have been shown to produce neurotoxic effects in animals.
Pharmacokinetics
Absorption – Following oral administration the bioavailability of methadone ranges between 36 to 100% and peak plasma concentrations are achieved between 1 to 7.5 hours. Dose proportionality of methadone pharmacokinetics is not known. However, after administration of daily oral doses ranging from 10 to 225 mg, the steady-state plasma concentrations ranged between 65 to 630 ng/mL and the peak concentrations ranged between 124 to 1255 ng/mL. Effect of food on the bioavailability of methadone has not been evaluated.
Distribution – Methadone is a lipophilic drug and the steady-state volume of distribution ranges between 1.0 to 8.0 L/kg. In plasma, methadone is predominantly bound to α-acid glycoprotein (85% to 90%). Methadone is secreted in saliva, breast milk, amniotic fluid and umbilical cord plasma.
Metabolism – Methadone is primarily metabolized by N-demethylation to an inactive metabolite, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidene (EDDP). Cytochrome P450 enzymes, primarily CYP3A4, CYP2B6, and CYP2C19 and to a lesser extent CYP2C9 and CYP2D6, are responsible for conversion of methadone to EDDP and other inactive metabolites, which are excreted mainly in the urine.
Excretion – The elimination of methadone is mediated by extensive biotransformation, followed by renal and fecal excretion. Published reports indicate that after multiple dose administration the terminal half-life (T1/2) was highly variable and ranged between 8 to 59 hours in different studies. Since methadone is lipophilic, it has been known to persist in the liver and other tissues. The slow release from the liver and other tissues may prolong the duration of methadone action despite low plasma concentrations.
Pharmacokinetics in Special Populations
Pregnancy – The disposition of oral methadone has been studied in approximately 30 pregnant patients in 2nd and 3rd trimesters. Elimination of methadone was significantly changed in pregnancy. Total body clearance of methadone was increased in pregnant patients compared to the same patients postpartum or to non-pregnant opioid-dependent women. The terminal half-life of methadone is decreased during 2nd and 3rd trimesters. The decrease in plasma half-life and increased clearance of methadone resulting in lower methadone trough levels during pregnancy can lead to withdrawal symptoms in some pregnant patients. The dosage may need to be increased or the dosing interval decreased in pregnant patients receiving methadone (see PRECAUTIONS, Pregnancy, Labor and Delivery, and DOSAGE AND ADMINISTRATION).
Renal Impairment – Methadone pharmacokinetics have not been extensively evaluated in patients with renal insufficiency. Unmetabolized methadone and its metabolites are excreted in urine to a variable degree. Methadone is a basic (pKa=9.2) compound and the pH of the urinary tract can alter its disposition in plasma. Urine acidification has been shown to increase renal elimination of methadone. Forced diuresis, peritoneal dialysis, hemodialysis, or charcoal hemoperfusion have not been established as beneficial for increasing the elimination of methadone or its metabolites.
Drug Interactions (see PRECAUTIONS, Drug Interactions)
Methadone undergoes hepatic N-demethylation by cytochrome P-450 isoforms, principally CYP3A4, CYP2B6, CYP2C19, and to a lesser extent by CYP2C9 and CYP2D6. Coadministration of methadone with inducers of these enzymes may result in more rapid methadone metabolism, and potentially, decreased effects of methadone. Conversely, administration with CYP inhibitors may reduce metabolism and potentiate methadone's effects. Pharmacokinetics of methadone may be unpredictable when coadministered with drugs that are known to both induce and inhibit CYP enzymes. Although antiretroviral drugs such as efavirenz, nelfinavir, nevirapine, ritonavir, lopinavir + ritonavir combination are known to inhibit some CYPs, they are shown to reduce the plasma levels of methadone, possibly due to their CYP induction activity. Therefore, drugs administered concomitantly with methadone should be evaluated for interaction potential; clinicians are advised to evaluate individual response to drug therapy before making a dosage adjustment.
-
INDICATIONS AND USAGE
- For the treatment of moderate to severe pain not responsive to non-narcotic analgesics.
- For detoxification treatment of opioid addiction (heroin or other morphine-like drugs).
- For maintenance treatment of opioid addiction (heroin or other morphine-like drugs), in conjunction with appropriate social and medical services.
Note – Outpatient maintenance and outpatient detoxification treatment may be provided only by Opioid Treatment Programs (OTPs) certified by the Federal Substance Abuse and Mental Health Services Administration (SAMHSA) and registered by the Drug Enforcement Administration (DEA). This does not preclude the maintenance treatment of a patient with concurrent opioid addiction who is hospitalized for conditions other than opioid addiction and who requires temporary maintenance during the critical period of his/her stay, or of a patient whose enrollment has been verified in a program which has been certified for maintenance treatment with methadone.
-
CONTRAINDICATIONS
Methadone is contraindicated in patients with a known hypersensitivity to methadone hydrochloride or any other ingredient in methadone hydrochloride tablets.
Methadone is contraindicated in any situation where opioids are contraindicated such as: patients with respiratory depression (in the absence of resuscitative equipment or in unmonitored settings), and in patients with acute bronchial asthma or hypercarbia.
Methadone is contraindicated in any patient who has or is suspected of having a paralytic ileus.
-
WARNINGS
Respiratory Depression, Incomplete Cross-Tolerance, and Iatrogenic Overdose
Respiratory depression is the chief hazard associated with methadone hydrochloride administration. Methadone's peak respiratory depressant effects typically occur later, and persist longer than its peak analgesic effects, particularly during the initial dosing period. These characteristics can contribute to cases of iatrogenic overdose, particularly during treatment initiation or dose titration.
Patients tolerant to other opioids may be incompletely tolerant to methadone. Incomplete cross-tolerance is of particular concern for patients tolerant to other mu-opioid agonists who are being converted to treatment with methadone, thus making determination of dosing during opioid treatment conversion complex. Deaths have been reported during conversion from chronic, high-dose treatment with other opioid agonists. Therefore, it is critical to understand the pharmacokinetics of methadone when converting patients from other opioids (see DOSAGE AND ADMINISTRATION, Table 1, for appropriate conversion schedules). A high degree of "opioid tolerance" does not eliminate the possibility of methadone overdose, iatrogenic or otherwise.
Respiratory depression is of particular concern in elderly or debilitated patients as well as in those suffering from conditions accompanied by hypoxia or hypercapnia when even moderate therapeutic doses may dangerously decrease pulmonary ventilation.
Methadone should be administered with extreme caution to patients with conditions accompanied by hypoxia, hypercapnia, or decreased respiratory reserve such as: asthma, chronic obstructive pulmonary disease or cor pulmonale, severe obesity, sleep apnea syndrome, myxedema, kyphoscoliosis, and CNS depression or coma. In these patients, even usual therapeutic doses of methadone may decrease respiratory drive while simultaneously increasing airway resistance to the point of apnea. Alternative, non-opioid analgesics should be considered, and methadone should be used at the lowest effective dose and only under careful medical supervision.
Cardiac Conduction Effects
Laboratory studies, both in vivo and in vitro, have demonstrated that methadone inhibits cardiac potassium channels and prolongs the QT interval. Cases of QT interval prolongation and serious arrhythmia (torsades de pointes) have been observed during treatment with methadone. These cases appear to be more commonly associated with, but not limited to, higher dose treatment (> 200 mg/day). Most cases involve patients being treated for pain with large, multiple daily doses of methadone, although cases have been reported in patients receiving doses commonly used for maintenance treatment of opioid addiction. In most of the cases seen at typical maintenance doses, concomitant medications and/or clinical conditions such as hypokalemia were noted as contributing factors. However, the evidence strongly suggests that methadone possesses the potential for adverse cardiac conduction effects in some patients.
Methadone should be administered with particular caution to patients already at risk for development of prolonged QT interval (e.g., cardiac hypertrophy, concomitant diuretic use, hypokalemia, hypomagnesemia). Careful monitoring is recommended when using methadone in patients with a history of cardiac conduction abnormalities, those taking medications affecting cardiac conduction, and in other cases where history or physical exam suggest an increased risk of dysrhythmia. QT prolongation has also been reported in patients with no prior cardiac history who have received high doses of methadone. Patients developing QT prolongation while on methadone treatment should be evaluated for the presence of modifiable risk factors, such as concomitant medications with cardiac effects, drugs which might cause electrolyte abnormalities, and drugs which might act as inhibitors of methadone metabolism. For use of methadone to treat pain, the risk of QT prolongation and development of dysrhythmias should be weighed against the benefit of adequate pain management and the availability of alternative therapies.
Methadone treatment for analgesic therapy in patients with acute or chronic pain should only be initiated if the potential analgesic or palliative care benefit of treatment with methadone has been considered to outweigh the risk of QT prolongation that has been reported with high doses of methadone.
The use of methadone in patients already known to have a prolonged QT interval has not been systematically studied.
In using methadone an individualized benefit to risk assessment should be carried out and should include evaluation of patient presentation and complete medical history. For patients judged to be at risk, careful monitoring of cardiovascular status, including QT prolongation and dysrhythmias and those described previously should be performed.
Misuse, Abuse, and Diversion of Opioids
Methadone is a mu-agonist opioid with an abuse liability similar to that of morphine and is a Schedule II controlled substance. Methadone, like morphine and other opioids used for analgesia, has the potential for being abused and is subject to criminal diversion.
Methadone can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing methadone hydrochloride tablets in situations where the clinician is concerned about an increased risk of misuse, abuse, or diversion.
Concerns about abuse, addiction, and diversion should not prevent the proper management of pain.
Healthcare professionals should contact their State Professional Licensing Board, or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
Interactions with Other CNS Depressants
Patients receiving other opioid analgesics, general anesthetics, phenothiazines, other tranquilizers, sedatives, hypnotics or other CNS depressants (including alcohol) concomitantly with methadone may experience respiratory depression, hypotension, profound sedation, or coma (see PRECAUTIONS).
Interactions with Alcohol and Drugs of Abuse
Methadone may be expected to have additive effects when used in conjunction with alcohol, other opioids, or illicit drugs that cause central nervous system depression. Deaths associated with illicit use of methadone frequently have involved concomitant benzodiazepine abuse.
Head Injury and Increased Intracranial Pressure
The respiratory depressant effects of opioids and their capacity to elevate cerebrospinal-fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a pre-existing increase in intracranial pressure. Furthermore, opioids produce effects which may obscure the clinical course of patients with head injuries. In such patients, methadone must be used with caution, and only if it is deemed essential.
-
DRUG ABUSE AND DEPENDENCE
Methadone hydrochloride tablets contain methadone, a mu-agonist opioid with an abuse liability similar to other opioid agonists and is a Schedule II controlled substance. Methadone and other opioids used in analgesia can be abused and are subject to criminal diversion.
Abuse of methadone poses a risk of overdose and death. This risk is increased with concurrent abuse of methadone with alcohol and other substances. In addition, parenteral drug abuse is commonly associated with transmission of infectious diseases such as hepatitis and HIV.
Drug addiction is characterized by compulsive use, use for non-medical purposes, and continued use despite harm or risk of harm. Drug addiction is a treatable disease, utilizing a multi-disciplinary approach, but relapse is common.
“Drug-seeking” behavior is very common in addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated claims of lost prescriptions, tampering with prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s). “Doctor shopping” (visiting multiple prescribers) to obtain additional prescriptions is common among drug abusers and people suffering from untreated addiction. However, it should be important to note that preoccupation with achieving adequate pain relief can be appropriate behavior in a patient with poor pain control.
Physical Dependence and Tolerance
Abuse and addiction are separate and distinct from physical dependence and tolerance. Physicians should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of true addiction and is characterized by misuse for non-medical purposes, often in combination with other psychoactive substances. Methadone, like other opioids, has been diverted for non-medical use. Careful record-keeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Infants born to mothers physically dependent on opioids may also be physically dependent and may exhibit respiratory difficulties and withdrawal symptoms (see PRECAUTIONS, Pregnancy, Labor and Delivery).
-
PRECAUTIONS
General
When treating pain, methadone given on a fixed-dose schedule may have a narrow therapeutic index in certain patient populations, especially when combined with other drugs, and should be reserved for cases where the benefits of opioid analgesia with methadone outweigh the known potential risks of cardiac conduction abnormalities, respiratory depression, altered mental states and postural hypotension. Methadone should be used with caution in elderly and debilitated patients; patients who are known to be sensitive to central nervous system depressants, such as those with cardiovascular, pulmonary, renal, or hepatic disease; and in patients with comorbid conditions or concomitant medications which may predispose to dysrhythmia.
Selection of patients for treatment with methadone should be governed by the same principles that apply to the use of other opioids (see INDICATIONS AND USAGE). Physicians should individualize treatment in every case (see DOSAGE AND ADMINISTRATION), taking into account the high degree of interpatient variability in response to and metabolism of methadone.
Drug Interactions
In vitro results suggest that methadone undergoes hepatic N-demethylation by cytochrome P450 enzymes, principally CYP3A4, CYP2B6, CYP2C19 and to a lesser extent by CYP2C9 and CYP2D6. Coadministration of methadone with CYP inducers of these enzymes may result in a more rapid metabolism and potential for decreased effects of methadone, whereas administration with CYP inhibitors may reduce metabolism and potentiate methadone's effects. Although antiretroviral drugs such as efavirenz, nelfinavir, nevirapine, ritonavir, lopinavir + ritonavir combination are known to inhibit CYPs, they are shown to reduce the plasma levels of methadone, possibly due to their CYP induction activity. Therefore, drugs administered concomitantly with methadone should be evaluated for interaction potential; clinicians are advised to evaluate individual response to drug therapy.
Opioid Antagonists, Mixed Agonist/Antagonists, and Partial Agonists
As with other mu-agonists, patients maintained on methadone may experience withdrawal symptoms when given opioid antagonists, mixed agonist/antagonists, and partial agonists. Examples of such agents are naloxone, naltrexone, pentazocine, nalbuphine, butorphanol, and buprenorphine.
Anti-Retroviral Agents
Abacavir, amprenavir, efavirenz, nelfinavir, nevirapine, ritonavir, lopinavir + ritonavir combination – Coadministration of these anti-retroviral agents resulted in increased clearance or decreased plasma levels of methadone. Methadone-maintained patients beginning treatment with these antiretroviral drugs should be monitored for evidence of withdrawal effects and methadone dose should be adjusted accordingly.
Cytochrome P450 Inducers
Methadone-maintained patients beginning treatment with CYP3A4 inducers should be monitored for evidence of withdrawal effects and methadone dose should be adjusted accordingly. The following drug interactions were reported following coadministration of methadone with inducers of cytochrome P450 enzymes:
Rifampin – In patients well-stabilized on methadone, concomitant administration of rifampin resulted in a marked reduction in serum methadone levels and a concurrent appearance of withdrawal symptoms.
Phenytoin – In a pharmacokinetic study with patients on methadone maintenance therapy, phenytoin administration (250 mg b.i.d. initially for 1 day followed by 300 mg QD for 3 to 4 days) resulted in an approximately 50% reduction in methadone exposure and withdrawal symptoms occurred concurrently. Upon discontinuation of phenytoin, the incidence of withdrawal symptoms decreased and methadone exposure increased to a level comparable to that prior to phenytoin administration.
Cytochrome P450 Inhibitors
Since the metabolism of methadone is mediated primarily by CYP3A4 isozyme, coadministration of drugs that inhibit CYP3A4 activity may cause decreased clearance of methadone. The expected clinical results would be increased or prolonged opioid effects. Thus, methadone-treated patients coadministered strong inhibitors of CYP3A4, such as azole antifungal agents (e.g., ketoconazole) and macrolide antibiotics (e.g., erythromycin), with methadone should be carefully monitored and dosage adjustment should be undertaken if warranted. Some selective serotonin reuptake inhibitors (SSRIs) (e.g., sertraline, fluvoxamine) may increase methadone plasma levels upon coadministration with methadone and result in increased opiate effects and/or toxicity.
Voriconazole – Repeat dose administration of oral voriconazole (400 mg Q12h for 1 day, then 200 mg Q12h for 4 days) increased the Cmax and AUC of (R)-methadone by 31% and 47%, respectively, in subjects receiving a methadone maintenance dose (30 to 100 mg QD). The Cmax and AUC of (S)-methadone increased by 65% and 103%, respectively. Increased plasma concentrations of methadone have been associated with toxicity including QT prolongation. Frequent monitoring for adverse events and toxicity related to methadone is recommended during coadministration. Dose reduction of methadone may be needed.
Others
Monoamine Oxidase (MAO) Inhibitors – Therapeutic doses of meperidine have precipitated severe reactions in patients concurrently receiving monoamine oxidase inhibitors or those who have received such agents within 14 days. Similar reactions thus far have not been reported with methadone. However, if the use of methadone is necessary in such patients, a sensitivity test should be performed in which repeated small, incremental doses of methadone are administered over the course of several hours while the patient's condition and vital signs are under careful observation.
Potentially Arrhythmogenic Agents
Extreme caution is necessary when any drug known to have the potential to prolong the QT interval is prescribed in conjunction with methadone. Pharmacodynamic interactions may occur with concomitant use of methadone and potentially arrhythmogenic agents such as class I and III antiarrhythmics, some neuroleptics and tricyclic antidepressants, and calcium channel blockers.
Caution should also be exercised when prescribing methadone concomitantly with drugs capable of inducing electrolyte disturbances (hypomagnesemia, hypokalemia) that may prolong the QT interval. These drugs include diuretics, laxatives, and, in rare cases, mineralocorticoid hormones.
Interactions with Alcohol and Drugs of Abuse
Methadone may be expected to have additive effects when used in conjunction with alcohol, other opioids or CNS depressants, or with illicit drugs that cause central nervous system depression. Deaths have been reported when methadone has been abused in conjunction with benzodiazepines.
Anxiety – Since methadone as used by tolerant patients at a constant maintenance dosage does not act as a tranquilizer, patients who are maintained on this drug will react to life problems and stresses with the same symptoms of anxiety as do other individuals. The physician should not confuse such symptoms with those of narcotic abstinence and should not attempt to treat anxiety by increasing the dose of methadone. The action of methadone in maintenance treatment is limited to the control of narcotic withdrawal symptoms and is ineffective for relief of general anxiety.
Acute Pain – Maintenance patients on a stable dose of methadone who experience physical trauma, postoperative pain or other acute pain cannot be expected to derive analgesia from their existing dose of methadone. Such patients should be administered analgesics, including opioids, in doses that would otherwise be indicated for non-methadone-treated patients with similar painful conditions. Due to the opioid tolerance induced by methadone, when opioids are required for management of acute pain in methadone patients, somewhat higher and/or more frequent doses will often be required than would be the case for non-tolerant patients.
Risk of Relapse in Patients on Methadone Maintenance Treatment of Opioid Addiction
Abrupt opioid discontinuation can lead to development of opioid withdrawal symptoms (see PRECAUTIONS). Presentation of these symptoms have been associated with an increased risk of susceptible patients to relapse to illicit drug use and should be considered when assessing the risks and benefit of methadone use.
Tolerance and Physical Dependence
Tolerance is the need for increasing doses of opioids to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors). Physical dependence is manifested by withdrawal symptoms after abrupt discontinuation of a drug or upon administration of an antagonist. Physical dependence and/or tolerance are not unusual during chronic opioid therapy.
If methadone is abruptly discontinued in a physically dependent patient, an abstinence syndrome may occur. The opioid abstinence or withdrawal syndrome is characterized by some or all of the following: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other symptoms also may develop, including irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate.
In general, chronically administered methadone should not be abruptly discontinued.
Special-Risk Patients
Methadone should be given with caution and the initial dose reduced in certain patients, such as the elderly and debilitated and those with severe impairment of hepatic or renal function, hypothyroidism, Addison's disease, prostatic hypertrophy, or urethral stricture. The usual precautions appropriate to the use of parenteral opioids should be observed and the possibility of respiratory depression should always be kept in mind.
Information for Patients
- Patients should be cautioned that methadone, like all opioids, may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving or operating machinery.
- Patients should be cautioned that methadone, like other opioids, may produce orthostatic hypotension in ambulatory patients.
- Patients should be cautioned that alcohol and other CNS depressants may produce an additive CNS depression when taken with this product and should be avoided.
- Patients should be instructed to seek medical attention immediately if they experience symptoms suggestive of an arrhythmia (such as palpitations, dizziness, lightheadedness, or syncope) when taking methadone.
- Patients initiating treatment with methadone for opioid dependence should be reassured that the dose of methadone will “hold” for longer periods of time as treatment progresses.
- Patients seeking to discontinue methadone maintenance treatment of opioid dependence should be apprised of the high risk of relapse to illicit drug use associated with discontinuation of methadone maintenance treatment.
- Patients should be instructed to keep methadone in a secure place out of the reach of children and other household members. Accidental or deliberate ingestion by a child may cause respiratory depression that can result in death. Patients and their caregivers should be advised to discard unused methadone in such a way that individuals other than the patient for whom it was originally prescribed will not come in contact with the drug.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis – The results of carcinogenicity assessment in B6C2F1 mice and Fischer 344 rats following dietary administration of two doses of methadone HCl have been published. Mice consumed 15 mg/kg/day or 60 mg/kg/day methadone for two years. These doses were approximately 0.6 and 2.5 times a human daily oral dose of 120 mg/day on a body surface area basis (mg/m2). There was a significant increase in pituitary adenomas in female mice treated with 15 mg/kg/day but not with 60 mg/kg/day. Under the conditions of the assay, there was no clear evidence for a treatment-related increase in the incidence of neoplasms in male rats. Due to decreased food consumption in males at the high dose, male rats consumed 16 mg/kg/day and 28 mg/kg/day of methadone for two years. These doses were approximately 1.3 and 2.3 times a human daily oral dose of 120 mg/day, based on body surface area comparison. In contrast, female rats consumed 46 mg/kg/day or 88 mg/kg/day for two years. These doses were approximately 3.7 and 7.1 times a human daily oral dose of 120 mg/day, based on body surface area comparison. Under the conditions of the assay, there was no clear evidence for a treatment-related increase in the incidence of neoplasms in either male or female rats.
Mutagenesis – There are several published reports on the potential genetic toxicity of methadone. Methadone tested negative in tests for chromosome breakage and disjunction and sex-linked recessive lethal gene mutations in germ cells of Drosophila using feeding and injection procedures. In contrast, methadone tested positive in the in vivo mouse dominant lethal assay and the in vivo mammalian spermatogonial chromosome aberration test. Additionally, methadone tested positive in the E. coli DNA repair system and Neurospora crassa and mouse lymphoma forward mutation assays.
Fertility – Reproductive function in human males may be decreased by methadone treatment. Reductions in ejaculate volume and seminal vesicle and prostate secretions have been reported in methadone-treated individuals. In addition, reductions in serum testosterone levels and sperm motility, and abnormalities in sperm morphology have been reported. Published animal studies provide additional data indicating that methadone treatment of males can alter reproductive function. Methadone produces a significant regression of sex accessory organs and testes of male mice and rats. Additional data have been published indicating that methadone treatment of male rats (once a day for three consecutive days) increased embryolethality and neonatal mortality. Examination of uterine contents of methadone-naive female mice bred to methadone-treated mice indicated that methadone treatment produced an increase in the rate of preimplantation deaths in all post-meiotic states.
Pregnancy
Teratogenic Effects. Pregnancy Category C – There are no controlled studies of methadone use in pregnant women that can be used to establish safety. However, an expert review of published data on experiences with methadone use during pregnancy by the Teratogen Information System (TERIS) concluded that maternal use of methadone during pregnancy as part of a supervised, therapeutic regimen is unlikely to pose a substantial teratogenic risk (quantity and quality of data assessed as “limited to fair”). However, the data are insufficient to state that there is no risk (TERIS, last reviewed October, 2002). Pregnant women involved in methadone maintenance programs have been reported to have significantly improved prenatal care leading to significantly reduced incidence of obstetric and fetal complications and neonatal morbidity and mortality when compared to women using illicit drugs. Several factors complicate the interpretation of investigations of the children of women who take methadone during pregnancy. These include the maternal use of illicit drugs, other maternal factors such as nutrition, infection, and psychosocial circumstances, limited information regarding dose and duration of methadone use during pregnancy, and the fact that most maternal exposure appears to occur after the first trimester of pregnancy. In addition, reported studies generally compare the benefit of methadone to the risk of untreated addiction to illicit drugs; the relevance of these findings to pain patients prescribed methadone during pregnancy is unclear.
Methadone has been detected in amniotic fluid and cord plasma at concentrations proportional to maternal plasma and in newborn urine at lower concentrations than corresponding maternal urine.
A retrospective series of 101 pregnant, opiate-dependent women who underwent inpatient opiate detoxification with methadone did not demonstrate any increased risk of miscarriage in the 2nd trimester or premature delivery in the 3rd trimester.
Several studies have suggested that infants born to narcotic-addicted women treated with methadone during all or part of pregnancy have been found to have decreased fetal growth with reduced birth weight, length, and/or head circumference compared to controls. This growth deficit does not appear to persist into later childhood. However, children born to women treated with methadone during pregnancy have been shown to demonstrate mild but persistent deficits in performance on psychometric and behavioral tests.
Additional information on the potential risks of methadone may be derived from animal data. Methadone does not appear to be teratogenic in the rat or rabbit models. However, following large doses, methadone produced teratogenic effects in the guinea pig, hamster and mouse. One published study in pregnant hamsters indicated that a single subcutaneous dose of methadone ranging from 31 to 185 mg/kg (the 31 mg/kg dose is approximately 2 times a human daily oral dose of 120 mg/day on a mg/m2 basis) on day 8 of gestation resulted in a decrease in the number of fetuses per litter and an increase in the percentage of fetuses exhibiting congenital malformations described as exencephaly, cranioschisis, and “various other lesions”. The majority of the doses tested also resulted in maternal death. In another study, a single subcutaneous dose of 22 to 24 mg/kg methadone (estimated exposure was approximately equivalent to a human daily oral dose of 120 mg/day on a mg/m2 basis) administered on day 9 of gestation in mice also produced exencephaly in 11% of the embryos. However, no effects were reported in rats and rabbits at oral doses up to 40 mg/kg (estimated exposure was approximately 3 and 6 times, respectively, a human daily oral dose of 120 mg/day on a mg/m2 basis) administered during days 6 to 15 and 6 to 18, respectively.
Nonteratogenetic Effects – Babies born to mothers who have been taking opioids regularly prior to delivery may be physically dependent. Onset of withdrawal symptoms in infants is usually in the first days after birth. Withdrawal signs in the newborn include irritability and excessive crying, tremors, hyperactive reflexes, increased respiratory rate, increased stools, sneezing, yawning, vomiting, and fever. The intensity of the syndrome does not always correlate with the maternal dose or the duration of maternal exposure. The duration of the withdrawal signs may vary from a few days to weeks or even months. There is no consensus on the appropriate management of infant withdrawal.
There are conflicting reports on whether SIDS occurs with an increased incidence in infants born to women treated with methadone during pregnancy.
Abnormal fetal nonstress tests (NSTs) have been reported to occur more frequently when the test is performed 1 to 2 hours after a maintenance dose of methadone in late pregnancy compared to controls.
Published animal data have reported increased neonatal mortality in the offspring of male rodents that were treated with methadone prior to mating. In these studies, the female rodents were not treated with methadone, indicating paternally-mediated developmental toxicity. Specifically, methadone administered to the male rat prior to mating with methadone-naive females resulted in decreased weight gain in progeny after weaning. The male progeny demonstrated reduced thymus weights, whereas the female progeny demonstrated increased adrenal weights. Further, behavioral testing of these male and female progeny revealed significant differences in behavioral tests compared to control animals, suggesting that paternal methadone exposure can produce physiological and behavioral changes in progeny in this model. Other animal studies have reported that perinatal exposure to opioids including methadone alters neuronal development and behavior in the offspring. Perinatal methadone exposure in rats has been linked to alterations in learning ability, motor activity, thermal regulation, nociceptive responses and sensitivity to drugs. Additional animal data demonstrates evidence for neurochemical changes in the brains of methadone-treated offspring, including changes to the cholinergic, dopaminergic, noradrenergic and serotonergic systems. Additional studies demonstrated that methadone treatment of male rats for 21 to 32 days prior to mating with methadone-naive females did not produce any adverse effects, suggesting that prolonged methadone treatment of the male rat resulted in tolerance to the developmental toxicities noted in the progeny. Mechanistic studies in this rat model suggest that the developmental effects of “paternal” methadone on the progeny appear to be due to decreased testosterone production. These animal data mirror the reported clinical findings of decreased testosterone levels in human males on methadone maintenance therapy for opioid addiction and in males receiving chronic intraspinal opioids.
Clinical Pharmacology for Pregnancy – Pregnant women appear to have significantly lower trough plasma methadone concentrations, increased plasma methadone clearance, and shorter methadone half-life than after delivery. Dosage adjustment using higher doses or administering the daily dose in divided doses may be necessary in pregnant women treated with methadone (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
Methadone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Labor and Delivery
As with all opioids, administration of this product to the mother shortly before delivery may result in some degree of respiratory depression in the newborn, especially if higher doses are used. Methadone is not recommended for obstetric analgesia because its long duration of action increases the probability of respiratory depression in the newborn. Narcotics with mixed agonist-antagonist properties should not be used for pain control during labor in patients chronically treated with methadone as they may precipitate acute withdrawal.
Nursing Mothers
Methadone is secreted into human milk. The safety of breast-feeding while taking oral methadone is controversial. At maternal oral doses of 10 to 80 mg/day, methadone concentrations from 50 to 570 mcg/L in milk have been reported, which, in the majority of samples, were lower than maternal serum drug concentrations at steady state. Peak methadone levels in milk occur approximately 4 to 5 hours after an oral dose. Based on an average milk consumption of 150 mL/kg/day, an infant would consume approximately 17.4 mcg/kg/day which is approximately 2 to 3% of the oral maternal dose. Methadone has been detected in very low plasma concentrations in some infants whose mothers were taking methadone. Women on high-dose methadone maintenance, who are already breast-feeding, should be counseled to wean breast-feeding gradually in order to prevent neonatal abstinence syndrome.
Methadone-treated mothers considering nursing an opioid-naive infant should be counseled regarding the presence of methadone in breast milk.
Because of the potential for serious adverse reactions in nursing infants from methadone, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. In patients being treated for opioid dependence, this should include weighing the risk of methadone against the risk of maternal illicit drug use.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 18 years have not been established.
Accidental or deliberate ingestion by a child may cause respiratory depression that can result in death. Patients and caregivers should be instructed to keep methadone in a secure place out of the reach of children and to discard unused methadone in such a way that individuals other than the patient for whom it was originally prescribed will not come in contact with the drug.
Geriatric Use
Clinical studies of methadone did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently compared to younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for elderly patients should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
Renal Impairment
The use of methadone has not been extensively evaluated in patients with renal insufficiency.
-
ADVERSE REACTIONS
Heroin Withdrawal
During the induction phase of methadone maintenance treatment, patients are being withdrawn from heroin and may therefore show typical withdrawal symptoms, which should be differentiated from methadone-induced side effects. They may exhibit some or all of the following signs and symptoms associated with acute withdrawal from heroin or other opiates: lacrimation, rhinorrhea, sneezing, yawning, excessive perspiration, goose-flesh, fever, chilliness alternating with flushing, restlessness, irritability, weakness, anxiety, depression, dilated pupils, tremors, tachycardia, abdominal cramps, body aches, involuntary twitching and kicking movements, anorexia, nausea, vomiting, diarrhea, intestinal spasms, and weight loss.
Initial Administration
The initial methadone dose should be carefully titrated to the individual. Too rapid titration for the patient's sensitivity is more likely to produce adverse effects.
The major hazards of methadone are respiratory depression and, to a lesser degree, systemic hypotension. Respiratory arrest, shock, cardiac arrest, and death have occurred.
The most frequently observed adverse reactions include lightheadedness, dizziness, sedation, nausea, vomiting, and sweating. These effects seem to be more prominent in ambulatory patients and in those who are not suffering severe pain. In such individuals, lower doses are advisable.
Other adverse reactions include the following:
Body as a Whole – asthenia (weakness), edema, headache
Cardiovascular – arrhythmias, bigeminal rhythms, bradycardia, cardiomyopathy, ECG abnormalities, extrasystoles, flushing, heart failure, hypotension, palpitations, phlebitis, QT interval prolongation, syncope, T-wave inversion, tachycardia, torsade de pointes, ventricular fibrillation, ventricular tachycardia
Digestive – abdominal pain, anorexia, biliary tract spasm, constipation, dry mouth, glossitis
Hematologic and Lymphatic – reversible thrombocytopenia has been described in opioid addicts with chronic hepatitis
Metabolic and Nutritional – hypokalemia, hypomagnesemia, weight gain
Nervous – agitation, confusion, disorientation, dysphoria, euphoria, insomnia, seizures
Respiratory – pulmonary edema, respiratory depression
Skin and Appendages – pruritis, urticaria, other skin rashes, and rarely, hemorrhagic urticaria
Special Senses – hallucinations, visual disturbances
Urogenital – amenorrhea, antidiuretic effect, reduced libido and/or potency, urinary retention or hesitancy
Maintenance on a Stabilized Dose – During prolonged administration of methadone, as in a methadone maintenance treatment program, there is usually a gradual, yet progressive, disappearance of side effects over a period of several weeks. However, constipation and sweating often persist.
-
DRUG ABUSE AND DEPENDENCE
Methadone contains methadone, a mu-agonist opioid with an abuse liability similar to other opioid agonists and is a Schedule II controlled substance. Methadone and other opioids used in analgesia have the potential for being abused and are subject to criminal diversion.
Abuse
Drug addiction is characterized by compulsive use, use for non-medical purposes, and continued use despite harm or risk of harm. Drug addiction is a treatable disease, utilizing a multi-disciplinary approach, but relapse is common.
“Drug-seeking” behavior is very common in addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated claims of lost prescriptions, tampering with prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s). “Doctor shopping” (visiting multiple prescribers) to obtain additional prescriptions is common among drug abusers and people suffering from untreated addiction. However, it should be important to note that preoccupation with achieving adequate pain relief can be appropriate behavior in a patient with poor pain control.
Physical Dependence and Tolerance
Abuse and addiction are separate and distinct from physical dependence and tolerance. Physicians should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of true addiction and is characterized by misuse for non-medical purposes, often in combination with other psychoactive substances. Methadone, like other opioids, has been diverted for non-medical use. Careful record-keeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Infants born to mothers physically dependent on opioids may also be physically dependent and may exhibit respiratory difficulties and withdrawal symptoms (see PRECAUTIONS, Pregnancy, Labor and Delivery).
-
OVERDOSAGE
Signs and Symptoms
Serious overdosage of methadone is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, maximally constricted pupils, skeletal-muscle flaccidity, cold and clammy skin, and sometimes, bradycardia and hypotension. In severe overdosage, particularly by the intravenous route, apnea, circulatory collapse, cardiac arrest, and death may occur.
Treatment
Primary attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and institution of assisted or controlled ventilation. If a non-tolerant person, takes a large dose of methadone, effective opioid antagonists are available to counteract the potentially lethal respiratory depression. The physician must remember, however, that methadone is a long-acting depressant (36 to 48 hours), whereas opioid antagonists act for much shorter periods (one to three hours). The patient must, therefore, be monitored continuously for recurrence of respiratory depression and may need to be treated repeatedly with the narcotic antagonist. If the diagnosis is correct and respiratory depression is due only to overdosage of methadone, the use of other respiratory stimulants is not indicated.
Opioid antagonists should not be administered in the absence of clinically significant respiratory or cardiovascular depression. In an individual physically dependent on opioids, the administration of the usual dose of an opioid antagonist may precipitate an acute withdrawal syndrome. The severity of this syndrome will depend on the degree of physical dependence and the dose of the antagonist administered. If antagonists must be used to treat serious respiratory depression in the physically dependent patient, the antagonist should be administered with extreme care and by titration with smaller than usual doses of the antagonist.
Intravenously administered naloxone or nalmefene may be used to reverse signs of intoxication. Because of the relatively short half-life of naloxone as compared with methadone, repeated injections may be required until the status of the patient remains satisfactory. Naloxone may also be administered by continuous intravenous infusion.
Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated.
-
DOSAGE AND ADMINISTRATION
Methadone differs from many other opioid agonists in several important ways. Methadone's pharmacokinetic properties, coupled with high interpatient variability in its absorption, metabolism, and relative analgesic potency, necessitate a cautious and highly individualized approach to prescribing. Particular vigilance is necessary during treatment initiation, during conversion from one opioid to another, and during dose titration.
While methadone's duration of analgesic action (typically 4 to 8 hours) in the setting of single-dose studies approximates that of morphine, methadone's plasma elimination half-life is substantially longer than that of morphine (typically 8 to 59 hours vs. 1 to 5 hours). Methadone's peak respiratory depressant effects typically occur later, and persist longer than its peak analgesic effects. Also, with repeated dosing, methadone may be retained in the liver and then slowly released, prolonging the duration of action despite low plasma concentrations. For these reasons, steady-state plasma concentrations, and full analgesic effects, are usually not attained until 3 to 5 days of dosing. Additionally, incomplete cross-tolerance between mu-opioid agonists makes determination of dosing during opioid conversion complex.
The complexities associated with methadone dosing can contribute to cases of iatrogenic overdose, particularly during treatment initiation and dose titration. A high degree of "opioid tolerance" does not eliminate the possibility of methadone overdose, iatrogenic or otherwise. Deaths have been reported during conversion to methadone from chronic, high-dose treatment with other opioid agonists and during initiation of methadone treatment of addiction in subjects previously abusing high doses of other agonists.
Treatment of Pain
Optimal methadone initiation and dose titration strategies for the treatment of pain have not been determined. Published equianalgesic conversion ratios between methadone and other opioids are imprecise, providing at best, only population averages that cannot be applied consistently to all patients. It should be noted that many commonly cited equianalgesia tables only present relative analgesic potencies of single opioid doses in non-tolerant patients, thus greatly underestimating methadone's analgesic potency, and its potential for adverse effects in repeated-dose settings. Regardless of the dose determination strategy employed, methadone is most safely initiated and titrated using small initial doses and gradual dose adjustments.
As with all opioid drugs, it is necessary to adjust the dosing regimen for each patient individually, taking into account the patient's prior analgesic treatment experience. The following dosing recommendations should only be considered as suggested approaches to what is actually a series of clinical decisions over time in the management of the pain of each individual patient. Prescribers should always follow appropriate pain management principles of careful assessment and ongoing monitoring.
In the selection of an initial dose of methadone hydrochloride tablets, attention should be given to the following:
- The total daily dose, potency and specific characteristics of the opioid the patient had been taking previously, if any;
- The relative potency estimate used to calculate an equianalgesic starting methadone dose, in particular, whether it is intended for use in acute or chronic methadone dosing;
- The patient's degree of opioid tolerance;
- The age, general condition and medical status of the patient;
- Concurrent medications, particularly other CNS and respiratory depressants;
- The type, severity and expected duration of the patient's pain;
- The acceptable balance between pain control and adverse side effects.
Initiation of Therapy in Opioid Non-Tolerant Patients
When oral methadone is used as the first analgesic in patients who are not already being treated with, and tolerant to, opioids, the usual oral methadone starting dose is 2.5 mg to 10 mg every 8 to 12 hours, slowly titrated to effect. More frequent administration may be required during methadone initiation in order to maintain adequate analgesia, and extreme caution is necessary to avoid overdosage, taking into account methadone's long elimination half-life.
Conversion from Parenteral Methadone to Oral Methadone
Conversion from parenteral methadone to oral methadone should initially use a 1:2 dose ratio (e.g., 5 mg parenteral methadone to 10 mg oral methadone).
Switching Patients to Methadone from other Chronic Opioids
Switching a patient from another chronically administered opioid to methadone requires caution due to the uncertainty of dose conversion ratios and incomplete cross-tolerance. Deaths have occurred in opioid tolerant patients during conversion to methadone.
Conversion ratios in many commonly used equianalgesic dosing tables do not apply in the setting of repeated methadone dosing. Although with single-dose administration the onset and duration of analgesic action, as well as the analgesic potency of methadone and morphine, are similar methadone's potency increases over time with repeated dosing. Furthermore, the conversion ratio between methadone and other opiates varies dramatically depending on baseline opiate (morphine equivalent) use as shown in the table below.
The dose conversion scheme below is derived from various consensus guidelines for converting chronic pain patients to methadone from morphine. Clinicians should consult published conversion guidelines to determine the equivalent morphine dose for patients converting from other opioids.
Table 1. Oral Morphine to Oral Methadone Conversion for Chronic Administration Total Daily Baseline Oral
Morphine DoseEstimated Daily Oral
Methadone Requirement as
Percent of Total Daily
Morphine Dose< 100 mg 20% to 30% 100 to 300 mg 10% to 20% 300 to 600 mg 8% to 12% 600 mg to 1000 mg 5% to 10% > 1000 mg < 5 % The total daily methadone dose derived from the table above may then be divided to reflect the intended dosing schedule (i.e., for administration every 8 hours, divide total daily methadone dose by 3).
Note – Equianalgesic methadone dosing varies not only between patients, but also within the same patient, depending on baseline morphine (or other opioid) dose. Table 1 has been included in order to illustrate this concept and to provide a safe starting point for opioid conversion. Methadone dosing should not be based solely on these tables. Methadone conversion and dose titration methods should always be individualized to account for the patient's prior opioid exposure, general medical condition, concomitant medication, and anticipated breakthrough medication use. The endpoint of titration is achievement of adequate pain relief, balanced against tolerability of opioid side effects. If a patient develops intolerable opioid related side effects, the methadone dose, or dosing interval, may need to be adjusted.
Dosage Adjustment During Pregnancy
Methadone clearance may be increased during pregnancy. Several small studies have demonstrated significantly lower trough methadone plasma concentrations and shorter methadone half-lives in women during their pregnancy compared to after their delivery. During pregnancy a woman's methadone dose may need to be increased, or their dosing interval decreased. Methadone should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Detoxification and Maintenance Treatment of Opiate Dependence
For detoxification and maintenance of opiate dependence methadone should be administered in accordance with the treatment standards cited in 42 CFR Section 8.12, including limitations on unsupervised administration. Induction/Initial Dosing
The initial methadone dose should be administered, under supervision, when there are no signs of sedation or intoxication, and the patient shows symptoms of withdrawal. Initially, a single dose of 20 to 30 mg of methadone will often be sufficient to suppress withdrawal symptoms. The initial dose should not exceed 30 mg. If same-day dosing adjustments are to be made, the patient should be asked to wait 2 to 4 hours for further evaluation, when peak levels have been reached. An additional 5 to 10 mg of methadone may be provided if withdrawal symptoms have not been suppressed or if symptoms reappear. The total daily dose of methadone on the first day of treatment should not ordinarily exceed 40 mg. Dose adjustments should be made over the first week of treatment based on control of withdrawal symptoms at the time of expected peak activity (e.g., 2 to 4 hours after dosing). Dose adjustment should be cautious; deaths have occurred in early treatment due to the cumulative effects of the first several days' dosing. Patients should be reminded that the dose will “hold” for a longer period of time as tissue stores of methadone accumulate.
Initial doses should be lower for patients whose tolerance is expected to be low at treatment entry. Loss of tolerance should be considered in any patient who has not taken opioids for more than 5 days. Initial doses should not be determined by previous treatment episodes or dollars spent per day on illicit drug use.
For Short-Term Detoxification
For patients preferring a brief course of stabilization followed by a period of medically supervised withdrawal, it is generally recommended that the patient be titrated to a total daily dose of about 40 mg in divided doses to achieve an adequate stabilizing level. Stabilization can be continued for 2 to 3 days, after which the dose of methadone should be gradually decreased. The rate at which methadone is decreased should be determined separately for each patient.
The dose of methadone can be decreased on a daily basis or at 2-day intervals, but the amount of intake should remain sufficient to keep withdrawal symptoms at a tolerable level. In hospitalized patients, a daily reduction of 20% of the total daily dose may be tolerated. In ambulatory patients, a somewhat slower schedule may be needed.
For Maintenance Treatment
Patients in maintenance treatment should be titrated to a dose at which opioid symptoms are prevented for 24 hours, drug hunger or craving is reduced, the euphoric effects of self-administered opioids are blocked or attenuated, and the patient is tolerant to the sedative effects of methadone. Most commonly, clinical stability is achieved at doses between 80 to 120 mg/day.
For Medically Supervised Withdrawal After a Period of Maintenance Treatment
There is considerable variability in the appropriate rate of methadone taper in patients choosing medically supervised withdrawal from methadone treatment. It is generally suggested that dose reductions should be less than 10% of the established tolerance or maintenance dose, and that 10 to 14-day intervals should elapse between dose reductions. Patients should be apprised of the high risk of relapse to illicit drug use associated with discontinuation of methadone maintenance treatment.
-
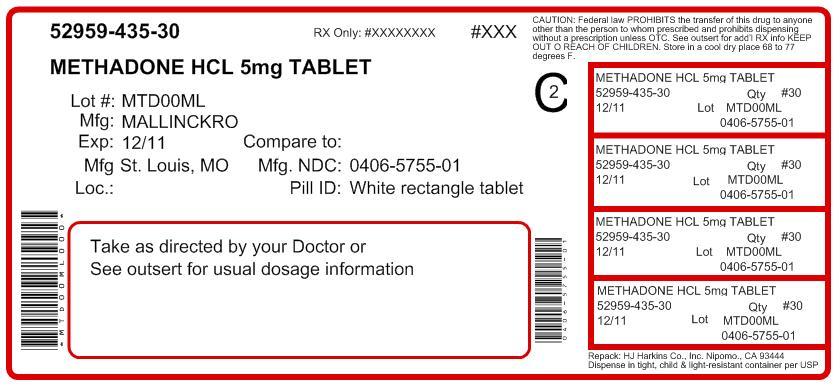
HOW SUPPLIED
Each 5 mg Methadone Hydrochloride Tablet USP contains 5 mg methadone hydrochloride USP. It is available as a white to off-white, modified rectangle shaped convex tablet, one side debossed with a score between “57” and “55”; on the other side.
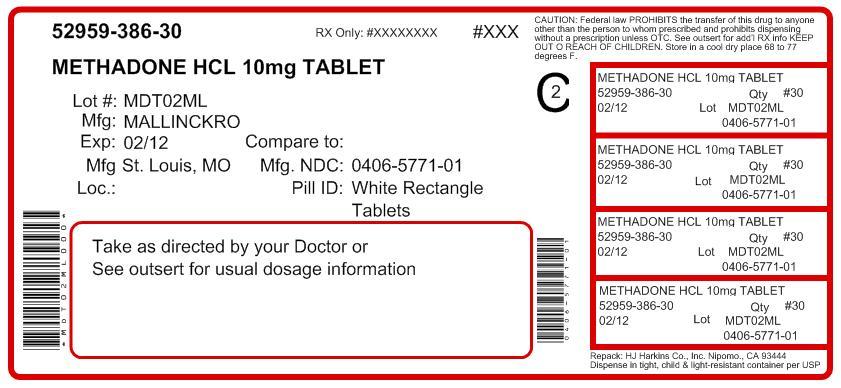
Bottles of 100 .................NDC: 0406-5755-01 Unit Dose (10 x 10)...........NDC: 0406-5755-62Each 10 mg Methadone Hydrochloride Tablet USP contains 10 mg methadone hydrochloride USP. It is available as a white to off-white, modified rectangle shaped convex tablet, one side debossed with a score between “57” and “71”; on the other side.
Bottles of 100 .................NDC: 0406-5771-01 Unit Dose (10 x 10)...........NDC: 0406-5771-62Dispense in a tight, light-resistant container (as defined in USP) with a child-resistant closure.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
COVIDIEN™
Mallinckrodt
Mallinckrodt Inc.,
Hazelwood, MO 63042 USA.
Rev 011309 -
PATIENT PACKAGE INSERT
CII
Patient Information
METHADONE HYDROCHLORIDE TABLETS USP
5 mg, 10 mg
WARNINGS:
Keep methadone hydrochloride tablets out of the reach of children. Accidental overdose by a child is a medical emergency and can result in death. If a child accidentally takes methadone hydrochloride tablets, get emergency help right away.
Do not take a higher dose of methadone hydrochloride tablets or take it more often than prescribed. This can lead to an overdose and possible death.
Read the Patient Information that comes with methadone hydrochloride tablets before you take it and each time you get a new prescription. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment. Share the important information in this leaflet with members of your household.
What is the Most Important Information I Should Know About Methadone Hydrochloride Tablets?
- Methadone hydrochloride tablets can cause life-threatening breathing problems which can lead to death. These problems are more likely to happen when methadone hydrochloride tablets are first started or in someone who is not already taking other narcotic (opioid) pain medicines.
- Breathing problems from methadone hydrochloride tablets may not happen right away after taking a dose. Sometimes breathing problems will happen a while after you take a dose, even after pain has returned. It is very important that you take methadone hydrochloride tablets exactly as your doctor has prescribed. Talk to your doctor about your pain. Your doctor can decide if your methadone hydrochloride tablets dose needs to be changed.
- Methadone hydrochloride tablets can cause life-threatening heartbeat problems that can lead to death. Most heartbeat problems have happened in people using large doses of methadone hydrochloride tablets for pain treatment. Some heartbeat problems have happened in people using smaller doses of methadone hydrochloride tablets for treatment of narcotic drug addiction.
What are Methadone Hydrochloride Tablets?
Methadone hydrochloride tablets are a prescription medicine that contains methadone, which is a narcotic pain medicine similar to morphine. Methadone hydrochloride tablets are a federally controlled substance (CII) because it is a strong opioid pain medicine that can be abused by people who abuse prescription medicines or street drugs.
- Prevent theft and misuse. Keep your methadone hydrochloride tablets in a safe place to protect them from theft. Never give methadone hydrochloride tablets to anyone else even if they have the same symptoms you have. It may harm them and even cause death. Selling or giving away this medicine is dangerous and against the law.
Methadone hydrochloride tablets are used:
- to treat moderate to severe pain in people that do not respond to non-narcotic pain medicines;
- to control withdrawal symptoms in patients being treated for narcotic drug addiction;
- for maintenance treatment of narcotic drug addiction along with other social and medical services. Stopping maintenance treatment of narcotic drug addiction with methadone hydrochloride tablets may result in a return to narcotic drug use.
Who Should Not Take Methadone Hydrochloride Tablets?
Do not take methadone hydrochloride tablets if you:
- have severe asthma or severe lung problems.
- have a blockage or obstruction in your intestines.
- are allergic to methadone or anything else in methadone hydrochloride tablets. See the end of this leaflet for a complete list of ingredients.
What Should I Tell my Doctor Before I Start Taking Methadone Hydrochloride Tablets?
Methadone hydrochloride tablets may not be right for you. Before starting methadone hydrochloride tablets, tell your doctor about all your medical and mental conditions including a history of drug or alcohol abuse or addiction.
Tell your doctor if you:
- are pregnant or plan to become pregnant. Methadone hydrochloride tablets may harm your unborn baby.
- are breast-feeding. Methadone hydrochloride tablets pass through your breast milk and may harm your baby. You should choose to use methadone hydrochloride tablets or breast-feed, but not both.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Some medicines may cause serious or life-threatening medical problems when taken with methadone hydrochloride tablets. Be especially careful about other medicines that may make you sleepy, such as other pain medicines, anti-depressant medicines, sleeping pills, anxiety medicines, antihistamines, or tranquilizers. Sometimes, the doses of certain medicines (including methadone hydrochloride tablets) may need to be changed if they are used together.
Do not take any medicine while using methadone hydrochloride tablets until you have first talked to your doctor or pharmacist. They will be able to tell you if it is safe to take other medicines while you are using methadone hydrochloride tablets.
Know the medicines you take. Keep a list of your medicines and show it to your doctor and pharmacist each time you get a new medicine.
How Should I Take Methadone Hydrochloride Tablets?
- Take methadone hydrochloride tablets exactly as prescribed. Follow your doctor's directions exactly. Your doctor may change your dose based on your reactions to the medicine. Do not change your dose unless your doctor tells you to change it. Do not take a higher dose of methadone hydrochloride tablets or take it more often than prescribed. This can lead to an overdose and possibly death.
- If you take too much methadone hydrochloride tablets or overdose, call 911 or your local emergency number right away.
- Review your medical conditions regularly with your doctor to determine if you still need methadone hydrochloride tablets, or if the dose needs to be adjusted.
- When starting treatment with methadone hydrochloride tablets for narcotic drug dependence, you should be aware that your dose of methadone will “hold” for longer periods of time as treatment goes on.
- Stopping methadone hydrochloride tablets. Ask your doctor for instructions on how to stop this medicine slowly to avoid uncomfortable symptoms. You should not stop taking methadone hydrochloride tablets all at once if you have been taking it for more than a few days.
- Tell all health professionals that treat you that you take methadone hydrochloride tablets.
- After stopping treatment with methadone hydrochloride tablets, flush the unused tablets down the toilet.
What Should I Avoid While Taking Methadone Hydrochloride Tablets?
- Do not drive, operate heavy machinery, or do other possible dangerous activities until you know how methadone hydrochloride tablets affect you. Methadone hydrochloride tablets can make you sleepy. Ask your doctor when it is okay to do these activities.
- Do not drink alcohol while using methadone hydrochloride tablets. It may increase the chance of having dangerous side effects.
- Do not take other medicines with methadone hydrochloride tablets without first talking with your doctor.
What are the Possible Side Effects of Methadone Hydrochloride Tablets?
-
Methadone hydrochloride tablets can cause life-threatening breathing and heart problems which can lead to death.
See
“What is the Most Important Information I Should Know About Methadone Hydrochloride Tablets?”
-
Call your doctor or get medical help right away if you:
- have trouble breathing.
- have extreme drowsiness and breathing slows down.
- have slow shallow breathing (little chest movement with breathing).
- fast or slowed heartbeat.
- feel faint, very dizzy, confused, have palpitations (irregular heartbeat) or any other unusual symptoms.
-
Call your doctor or get medical help right away if you:
These can be symptoms that you have taken too much (overdose of) methadone hydrochloride tablets, or the dose is too high for you. They can also be symptoms of a serious heart reaction. These symptoms can lead to serious problems or death if not treated right away.
- Methadone hydrochloride tablets can cause your blood pressure to drop. This can make you feel dizzy if you get up too fast from sitting or lying down.
- Methadone hydrochloride tablets can cause physical dependence. Do not stop taking methadone hydrochloride tablets or any other opioid without first talking to your doctor. You could become sick with uncomfortable withdrawal symptoms because your body has become used to these medicines. Talk to your doctor about slowly stopping methadone hydrochloride tablets to avoid getting sick with withdrawal symptoms. Physical dependency is not the same as drug addiction.
- For patients using methadone hydrochloride tablets for pain treatment, there is a chance of abuse or addiction with methadone hydrochloride tablets. The chance is higher if you are or have been addicted to or abused other medicines, street drugs, or alcohol, or if you have a history of mental problems.
Some common side effects of methadone hydrochloride tablets are lightheadedness, dizziness, drowsiness, nausea, vomiting and sweating. Other side effects include weakness, headache, constipation, itching, and dry mouth.
Talk to your doctor about any side effects that bother you or that do not go away.
These are not all the possible side effects of methadone hydrochloride tablets. For a complete list, ask your doctor or pharmacist.
How Should I Store Methadone Hydrochloride Tablets?
- Keep methadone hydrochloride tablets in a safe place away from children. Accidental use by a child is a medical emergency that can result in death. If a child accidentally takes methadone hydrochloride tablets, get emergency help right away.
- Keep methadone hydrochloride tablets at room temperature, 68° to 77°F (20° to 25°C).
- Always keep methadone hydrochloride tablets in a secure place to protect from theft.
- Dispose of any unused methadone hydrochloride tablets remaining from a prescription as soon as they are no longer needed. Unused tablets should be flushed down the toilet.
General Information About Methadone Hydrochloride Tablets
Medicines are sometimes prescribed for purposes other than those listed in patient information leaflet. Do not use methadone hydrochloride tablets for a condition for which it was not prescribed. Do not give methadone hydrochloride tablets to other people, even if they have the same symptoms you have. Methadone hydrochloride tablets can harm other people and even cause death. Sharing methadone hydrochloride tablets is against the law.
This leaflet summarizes the most important information about methadone hydrochloride tablets. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about methadone hydrochloride tablets that was written for healthcare professionals, or you can visit www.Mallinckrodt.com or call 1-800-778-7898.
What are the Ingredients in Methadone Hydrochloride Tablets?
Active Ingredient: methadone hydrochloride USP
Inactive Ingredients: lactose monohydrate, magnesium stearate, microcrystalline cellulose and silicon dioxide.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
-
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 5 mg Bottle
NDC: 0406-5755-01
100 TABLETS
METHADONE HYDROCHLORIDE
TABLETS USP
CII
5 mg
Each tablet contains:
Methadone Hydrochloride USP. . . . . 5 mg
Rx only
Mallinckrodt
-
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 10 mg Bottle
NDC: 0406-5771-01
100 TABLETS
METHADONE HYDROCHLORIDE
TABLETS USP
CII
10 mg
Each tablet contains:
Methadone Hydrochloride USP. . . . 10 mg
Rx only
MallinckrodtRepacked by:
H.J. Harkins Company, Inc.
Nipomo, CA 93444
-
INGREDIENTS AND APPEARANCE
METHADONE HYDROCHLORIDE
methadone hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52959-435(NDC:0406-5771) Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHADONE HYDROCHLORIDE (UNII: 229809935B) (METHADONE - UNII:UC6VBE7V1Z) METHADONE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color white (off-white) Score 2 pieces Shape RECTANGLE Size 9mm Flavor Imprint Code 57;55;M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52959-435-30 30 in 1 BOTTLE 2 NDC: 52959-435-60 60 in 1 BOTTLE 3 NDC: 52959-435-90 90 in 1 BOTTLE 4 NDC: 52959-435-02 120 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040517 05/19/2009 METHADONE HYDROCHLORIDE
methadone hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52959-386(NDC:0406-5755) Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHADONE HYDROCHLORIDE (UNII: 229809935B) (METHADONE - UNII:UC6VBE7V1Z) METHADONE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color white (off-white) Score 2 pieces Shape RECTANGLE Size 11mm Flavor Imprint Code 57;71;M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52959-386-30 30 in 1 BOTTLE 2 NDC: 52959-386-60 60 in 1 BOTTLE 3 NDC: 52959-386-90 90 in 1 BOTTLE 4 NDC: 52959-386-02 120 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040517 05/19/2009 Labeler - H.J. Harkins Company Inc. (147681894) Establishment Name Address ID/FEI Business Operations Mallinckrodt Inc. 957414238 manufacture, analysis
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.