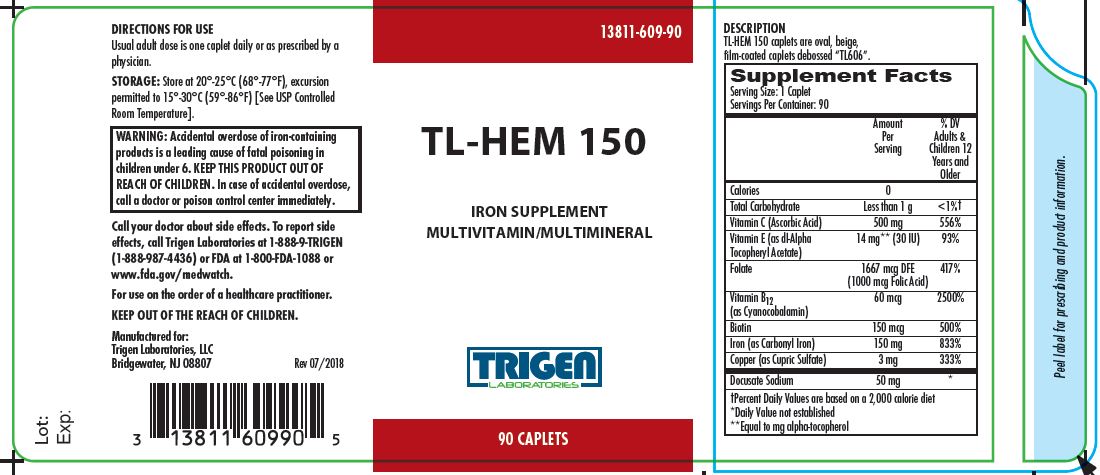
TL-HEM 150- ascorbic acid, .alpha.-tocopherol acetate, dl-, folic acid, cyanocobalamin, biotin, iron, cupric sulfate, and docusate sodium tablet, film coated
TL-HEM 150 by
Drug Labeling and Warnings
TL-HEM 150 by is a Other medication manufactured, distributed, or labeled by Trigen Laboratories, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SUPPLEMENT FACTS TABLE
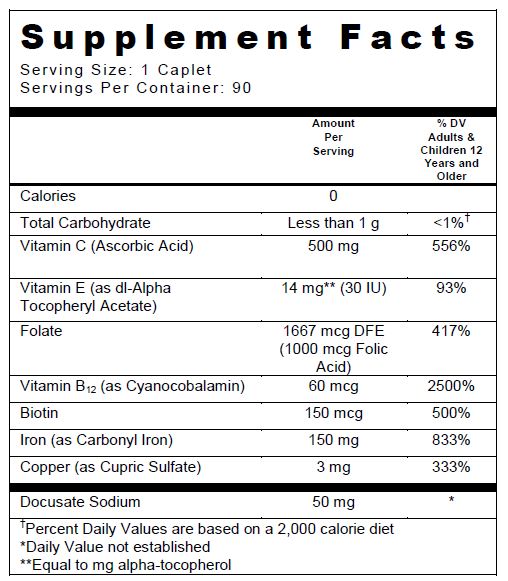
Other Ingredients: Microcrystalline Cellulose, Coating (Polyvinyl Alcohol, Titanium Dioxide, Polyethylene Glycol, Talc, FD&C Yellow #6 Lake, FD&C Blue #2 Lake), Tripotassium Citrate, Citric Acid, Povidone K30, Acacia, Stearic Acid, Fumed Silica, and Magnesium Stearate.
TL-HEM 150 is a hematinic multivitamin/multimineral dietary supplement used to improve the nutritional status of patients with iron deficiency. - CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 1.0 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations remain progressive. Do not exceed recommended daily dose. Some patients affected with pernicious anemia may not respond to orally administered Vitamin B12 with intrinsic factor concentrate and there is no known way to predict which patients will respond. Periodic clinical assessment of pernicious anemia patients by a physician are recommended. If any symptoms of intolerance to TL-HEM 150, including but not limited to nausea, vomiting, abdominal cramps or rash occur, the drug should be discontinued, as determined by a physician.
Biotin levels higher than the recommended daily allowance may cause interference with some laboratory tests, including cardiovascular diagnostic tests (e.g. troponin) and hormone test, and may lead to incorrect test results. Tell your healthcare provider about all prescription and over-the-counter medicines, vitamins, and dietary supplements that you take, including biotin.
Pediatric Use: Safety and effectiveness in pediatric patients have not been established.
Geriatric Use: Safety and effectiveness in elderly patients have not been established. -
ADVERSE REACTIONS
Adverse reactions with iron therapy may include constipation, diarrhea, nausea, vomiting, dark stools and abdominal pain. Skin rashes of various types may occur. Such reactions may necessitate temporary or permanent changes in dosage or usage. Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
- DESCRIPTION
- DIRECTIONS FOR USE
- HOW SUPPLIED
- STORAGE
-
HEALTH CLAIM
KEEP OUT OF REACH OF CHILDREN.
For use on the order of a healthcare practitioner.
Call your doctor about side effects. To report side effects, call Trigen Laboratories at 1-888-9-TRIGEN (1-888-987-4436) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Customer Service: 1-888-987-4436 Rev. 07/2018
Manufactured for:
Trigen Laboratories, LLC
Bridgewater, NJ 08807

- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TL-HEM 150
ascorbic acid, .alpha.-tocopherol acetate, dl-, folic acid, cyanocobalamin, biotin, iron, cupric sulfate, and docusate sodium tablet, film coatedProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:13811-609 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 500 mg .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 30 [iU] FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1000 ug CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 60 ug BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 150 ug IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 150 mg CUPRIC SULFATE (UNII: LRX7AJ16DT) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 3 mg DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POVIDONE K30 (UNII: U725QWY32X) ACACIA (UNII: 5C5403N26O) STEARIC ACID (UNII: 4ELV7Z65AP) MAGNESIUM STEARATE (UNII: 70097M6I30) POTASSIUM CITRATE ANHYDROUS (UNII: 86R1NVR0HW) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:13811-609-90 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 04/01/2018 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color imprint scoring 1 shape size (solid drugs) 19 mm Labeler - Trigen Laboratories, LLC (830479668)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.