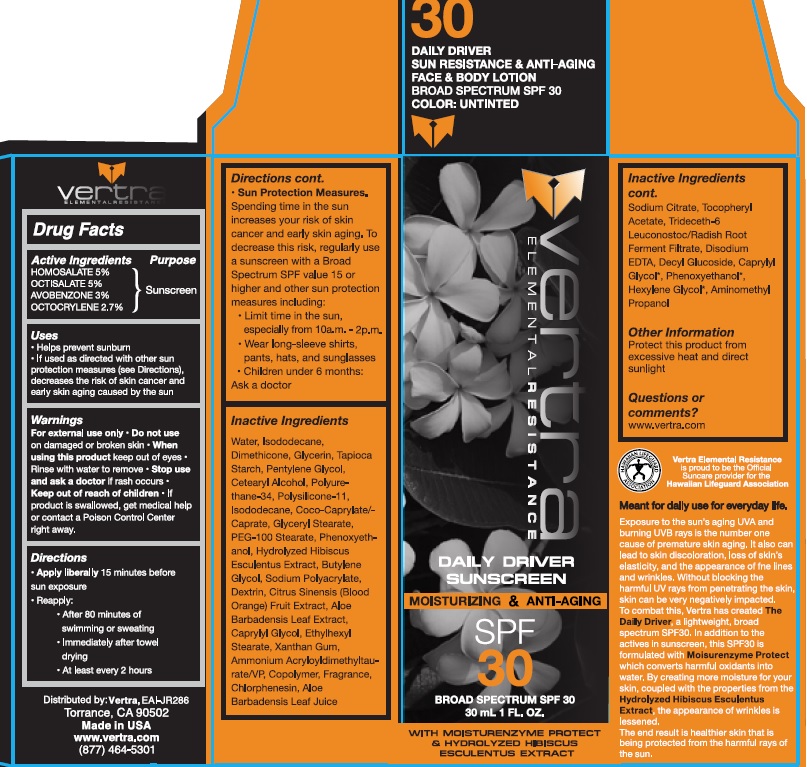
Vertra Daily Driver Sunscreen Moisturizer and Anti-Aging SPF30
Vertra Element Resistance Daily Driver by
Drug Labeling and Warnings
Vertra Element Resistance Daily Driver by is a Otc medication manufactured, distributed, or labeled by EAi-JR286, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VERTRA ELEMENT RESISTANCE DAILY DRIVER SPF30- homosalate, octisalate, avobenzone, octocrylene lotion
EAi-JR286, INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Vertra Daily Driver Sunscreen Moisturizer and Anti-Aging SPF30
Uses
- Helps prevent Sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused byt the sun
Directions
Apply Liberally 15 minutes before sun exposure
Reapply:
after 80 minutes of swimming or sweating
Immediately after towel drying
At least every 2 hours
Sun Protection Measures
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value 15 or higher and other sun protection measuers including
limit time in the sun, especially from 10 a.m.-2p.m.
wear long-sleeve shirts, pants, hats and sunglasses
Children under 6 months: Ask a doctor.
Inactive Ingredients
Aloe Barbadensis (Aloe Vera) Leaf Juice, Aloe Barbadensis Leaf Extract, Aminomethyl Propanol, Ammonium Acryloyldimethyltaurate/VP Copolymer, Butylene Glycol, Caprylyl Glycol, Cetearyl Alcohol, Chlorphenesin, Citrus Sinensis (Blood Orange) Fruit Extract, Coco Caprylate/Caprate, Decyl Glucoside, Dextrin, Dimethicone, Disodium EDTA, Ethylhexyl Stearate, Fragrance, Glycerin, Glyceryl Stearate,
Hydrolyzed Hibiscus Esculentus Extract, Isododecane, PEG-100 Stearate, Pentylene Glycol, Phenoxyethanol, Polysilicone-11, Polyurethane-34, Sodium Citrate, Sodium Polyacrylate, Tapioca Starch Polymethylsilsesquioxane, Tocopheryl Acetate, Trideceth 6, Water,
Xanthan Gum
| VERTRA ELEMENT RESISTANCE DAILY DRIVER
SPF30
homosalate, octisalate, avobenzone, octocrylene lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - EAi-JR286, INC (827896718) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.