ERY-TAB- erythromycin tablet, delayed release
ERY-TAB by
Drug Labeling and Warnings
ERY-TAB by is a Prescription medication manufactured, distributed, or labeled by Carilion Materials Management. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
ERY-TAB (erythromycin delayed-release tablets) is an antibacterial product containing erythromycin base in a specially enteric-coated tablet. The coating protects the antibiotic from the inactivating effects of gastric acidity and permits efficient absorption of the antibiotic in the small intestine. ERY-TAB tablets for oral administration are available in three dosage strengths, each white oval tablet containing either 250 mg, 333 mg, or 500 mg of erythromycin as the free base. ERY-TAB tablets comply with ®®®USP Dissolution Test 1.
Erythromycin is produced by a strain of (formerly ) and belongs to the macrolide group of antibiotics. It is basic and readily forms salts with acids. Erythromycin is a white to off-white powder, slightly soluble in water, and soluble in alcohol, chloroform, and ether. Erythromycin is known chemically as (3R*, 4S*, 5S*, 6R*, 7R*, 9R*, 11R*, 12R*,13S*, 14R*)-4-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α -L- -hexopyranosyl)oxy]-14-ethyl-7,12,13-trihdroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-(dimethylamino)-β-D- -hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione. The molecular formula is C H NO , and the molecular weight is 733.94. The structural formula is: Saccharopolyspora erythraeaStreptomyces erythraeusriboxylo376713
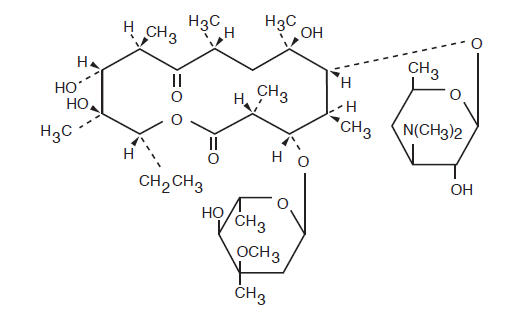
Inactive Ingredients
Ammonium hydroxide, colloidal silicon dioxide, croscarmellose sodium, crospovidone, diacetylated monoglycerides, hydroxypropyl cellulose, hypromellose, hypromellose phthalate, magnesium stearate, microcrystalline cellulose, povidone, propylene glycol, sodium citrate, sorbitan monooleate, talc, and titanium dioxide.
-
CLINICAL PHARMACOLOGY
Orally administered erythromycin base and its salts are readily absorbed in the microbiologically active form. Interindividual variations in the absorption of erythromycin are, however, observed, and some patients do not achieve optimal serum levels. Erythromycin is largely bound to plasma proteins. After absorption, erythromycin diffuses readily into most body fluids. In the absence of meningeal inflammation, low concentrations are normally achieved in the spinal fluid but the passage of the drug across the blood-brain barrier increases in meningitis. Erythromycin crosses the placental barrier, but fetal plasma levels are low. The drug is excreted in human milk. Erythromycin is not removed by peritoneal dialysis or hemodialysis.
In the presence of normal hepatic function, erythromycin is concentrated in the liver and is excreted in the bile; the effect of hepatic dysfunction on biliary excretion of erythromycin is not known. After oral administration, less than 5% of the administered dose can be recovered in the active form in the urine.
ERY-TAB tablets are coated with a polymer whose dissolution is pH dependent. This coating allows for minimal release of erythromycin in acidic environments, e.g., stomach. The tablets are designed for optimal drug release and absorption in the small intestine. In multiple-dose, steady-state studies, ERY-TAB tablets have demonstrated adequate drug delivery in both fasting and non-fasting conditions. Bioavailability data are available. ®®
Microbiology
Mechanism of Action
Erythromycin acts by inhibition of protein synthesis by binding 50S ribosomal subunits of susceptible organisms. It does not affect nucleic acid synthesis.
Mechanism of Resistance
The major route of resistance is modification of the 23S rNA in the 50S ribosomal subunit to insensitivity while efflux can also be significant.
Interactions with Other Antibiotics
Antagonism exists between erythromycin and clindamycin, lincomycin, and chloramphenicol. in vitro
Erythromycin has been shown to be active against most isolates of the following bacteria both and in clinical infections as described in the section. in vitroINDICATIONS AND USAGE
Gram-positive Bacteria
- Corynebacterium diphtheriae
- Corynebacterium minutissimum
- Listeria monocytogenes
- Staphylococcus aureus (resistant organisms may emerge during treatment)
- Streptococcus pneumoniae
- Streptococcus pyogenes
Gram-negative Bacteria
- Bordetella pertussis
- Haemophilus influenzae
- Legionella pneumophila
- Neisseria gonorrhoeae
Other Microorganisms
- Chlamydia trachomatis
- Entamoeba histolytica
- Mycoplasma pneumoniae
- Treponema pallidum
- Ureaplasma urealyticum
The following data are available, in vitrobut their clinical significance is unknown.
At least 90% of the following bacteria exhibit minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for erythromycin. However, the efficacy of erythromycin in treating clinical infections due to these bacteria has not been established in adequate and well controlled clinical trials. in vitro
Susceptibility Test Methods
When available the clinical microbiology laboratory should provide the results of susceptibility test results for antimicrobial drug products used in resident hospitals to the physician as periodic reports that describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting an antibacterial drug product for treatment. in vitro
Dilution Techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MIC's). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized test method (broth and/or agar). The MIC values should be interpreted according to criteria provided in . 1, 2Table 1
Diffusion techniques
Quantitative methods that require measurement of zone diameters can also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. The zone size provides an estimate of the susceptibility of bacteria to antimicrobial compounds. The zone size should be determined using a standardized test method. This procedure uses paper disks impregnated with 15 mcg erythromycin to test the susceptibility of microorganisms to erythromycin. The disc diffusion interpretive criteria are provided in . 2,3Table 1
Table 1. In Vitro Susceptibility Test Interpretive Criteria for Erythromycin Minimum Inhibitory Concentrations (mcg/mL)
Disk Diffusion (zone diameters in mm)
Pathogen S I R S I R Staphylococcus aureus ≤0.5 1-4 ≥8 ≥23 14-22 ≤13 Streptococcus pneumoniae ≤0.25 0.5 ≥1 ≥21 16-20 ≤15 Streptococcus pyogenes ≤0.25 0.5 ≥1 ≥21 16-20 ≤15 A report of "Susceptible" indicates that the antimicrobial is likely to inhibit growth of the pathogen if the antimicrobial compound reaches the concentrations at the site of infection necessary to inhibit growth of the pathogen. A report of "Intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug product is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that the antimicrobial is not likely to inhibit growth of the pathogen if the antimicrobial compound reaches the concentrations usually achievable at the infection site; other therapy should be selected.
Quality Control
Standardized susceptibility test procedures require the use of laboratory controls to monitor and ensure the accuracy and precision of supplies and reagents used in the assay, and the techniques of the individuals performing the test. Standard erythromycin powder should provide the following range of MIC values noted in . For the diffusion technique using the 15 mcg disk, the criteria in should be achieved. 1, 2, 3, 4Table 2Table 2
Table 2. Acceptable Quality Control Ranges for Erythromycin QC Strain Minimum Inhibitory Concentrations (mcg/mL)
Disk Diffusion (zone diameters in mm)
ATCC 29213 Staphylococcus aureus
0.25-1 NA ATCC 25923 Staphylococcus aureus
NA 22-30 ATCC 29212 Enterococcus faecalis
1-4 NA ATCC 49619 Streptococcus pneumoniae
0.03-0.12 25-30 -
INDICATIONS AND USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of ERY-TAB and other antibacterial drugs, ERY-TAB should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. ®®
ERY-TAB tablets are indicated in the treatment of infections caused by susceptible strains of the designated microorganisms in the diseases listed below: ®
Upper respiratory tract infections of mild to moderate degree caused by ; ; (when used concomitantly with adequate doses of sulfonamides, since many strains of are not susceptible to the erythromycin concentrations ordinarily achieved). (See appropriate sulfonamide labeling for prescribing information.) Streptococcus pyogenesStreptococcus pneumoniaeHaemophilus influenzaeH. influenzae
Lower respiratory tract infections of mild to moderate severity caused by or . Streptococcus pyogenesStreptococcus pneumoniae
Listeriosis caused by . Listeria monocytogenes
Respiratory tract infections due to . Mycoplasma pneumoniae
Skin and skin structure infections of mild to moderate severity caused by or (resistant staphylococci may emerge during treatment). Streptococcus pyogenesStaphylococcus aureus
Pertussis (whooping cough) caused by . Erythromycin is effective in eliminating the organism from the nasopharynx of infected individuals, rendering them noninfectious. Some clinical studies suggest that erythromycin may be helpful in the prophylaxis of pertussis in exposed susceptible individuals. Bordetella pertussis
Diphtheria: Infections due to , as an adjunct to antitoxin, to prevent establishment of carriers and to eradicate the organism in carriers. Corynebacterium diphtheriae
Erythrasma: In the treatment of infections due to . Corynebacterium minutissimum
Intestinal amebiasis caused by (oral erythromycins only). Extraenteric amebiasis requires treatment with other agents. Entamoeba histolytica
Acute pelvic inflammatory disease caused by : Erythrocin Lactobionate-I.V. (erythromycin lactobionate for injection, USP) followed by erythromycin base orally, as an alternative drug in treatment of acute pelvic inflammatory disease caused by in female patients with a history of sensitivity to penicillin. Patients should have a serologic test for syphilis before receiving erythromycin as treatment of gonorrhea and a follow-up serologic test for syphilis after 3 months. Neisseria gonorrhoeae®N. gonorrhoeae
Erythromycins are indicated for treatment of the following infections caused by : conjunctivitis of the newborn, pneumonia of infancy, and urogenital infections during pregnancy. When tetracyclines are contraindicated or not tolerated, erythromycin is indicated for the treatment of uncomplicated urethral, endocervical, or rectal infections in adults due to . Chlamydia trachomatisChlamydia trachomatis
When tetracyclines are contraindicated or not tolerated, erythromycin is indicated for the treatment of nongonococcal urethritis caused by . Ureaplasma urealyticum
Primary syphilis caused by . Erythromycin (oral forms only) is an alternative choice of treatment for primary syphilis in patients allergic to the penicillins. In treatment of primary syphilis, spinal fluid should be examined before treatment and as part of the follow-up after therapy. Treponema pallidum
Legionnaires' Disease caused by . Although no controlled clinical efficacy studies have been conducted, and limited preliminary clinical data suggest that erythromycin may be effective in treating Legionnaires' Disease. Legionella pneumophilain vitro
Prophylaxis
Prevention of Initial Attacks of Rheumatic Fever
Penicillin is considered by the American Heart Association to be the drug of choice in the prevention of initial attacks of rheumatic fever (treatment of Streptococcus pyogenes infections of the upper respiratory tract e.g., tonsillitis, or pharyngitis). Erythromycin is indicated for the treatment of penicillin-allergic patients. The therapeutic dose should be administered for ten days. 4
Prevention of Recurrent Attacks of Rheumatic Fever
Penicillin or sulfonamides are considered by the American Heart Association to be the drugs of choice in the prevention of recurrent attacks of rheumatic fever. In patients who are allergic to penicillin and sulfonamides, oral erythromycin is recommended by the American Heart Association in the long-term prophylaxis of streptococcal pharyngitis (for the prevention of recurrent attacks of rheumatic fever). 4
-
CONTRAINDICATIONS
Erythromycin is contraindicated in patients with known hypersensitivity to this antibiotic.
Erythromycin is contraindicated in patients taking terfenadine, astemizole, cisapride, pimozide, ergotamine, or dihydroergotamine. (See .) PRECAUTIONS – Drug Interactions
-
WARNINGS
Hepatotoxicity
There have been reports of hepatic dysfunction, including increased liver enzymes, and hepatocellular and/or cholestatic hepatitis, with or without jaundice, occurring in patients receiving oral erythromycin products.
QT Prolongation
Erythromycin has been associated with prolongation of the QT interval and infrequent cases of arrhythmia. Cases of torsades de pointes have been spontaneously reported during postmarketing surveillance in patients receiving erythromycin. Fatalities have been reported. Erythromycin should be avoided in patients with known prolongation of the QT interval, patients with ongoing proarrhythmic conditions such as uncorrected hypokalemia or hypomagnesemia, clinically significant bradycardia, and in patients receiving Class IA (quinidine, procainamide) or Class III (dofetilide, amiodarone, sotalol) antiarrhythmic agents. Elderly patients may be more susceptible to drug-associated effects on the QT interval.
Syphilis in Pregnancy
There have been reports suggesting that erythromycin does not reach the fetus in adequate concentration to prevent congenital syphilis. Infants born to women treated during pregnancy with oral erythromycin for early syphilis should be treated with an appropriate penicillin regimen.
Clostridium difficile Associated Diarrhea
associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ERY-TAB , and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of . Clostridium difficile®C. difficile
produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents. C. difficileC. difficile
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of , and surgical evaluation should be instituted as clinically indicated. C. difficileC. difficile
Drug Interactions
Serious adverse reactions have been reported in patients taking erythromycin concomitantly with CYP3A4 substrates. These include colchicine toxicity with colchicine; rhabdomyolysis with simvastatin, lovastatin, and atorvastatin; and hypotension with calcium channel blockers metabolized by CYP3A4 (e.g., verapamil, amlodipine, diltiazem) (see ). PRECAUTIONS - Drug Interactions
There have been post-marketing reports of colchicine toxicity with concomitant use of erythromycin and colchicine. This interaction is potentially life-threatening, and may occur while using both drugs at their recommended doses (see ). PRECAUTIONS - Drug Interactions
Rhabdomyolysis with or without renal impairment has been reported in seriously ill patients receiving erythromycin concomitantly with lovastatin. Therefore, patients receiving concomitant lovastatin and erythromycin should be carefully monitored for creatine kinase (CK) and serum transaminase levels. (See package insert for lovastatin.)
-
PRECAUTIONS
General
Prescribing ERY-TAB in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria. ®
Since erythromycin is principally excreted by the liver, caution should be exercised when erythromycin is administered to patients with impaired hepatic function. (See and .) CLINICAL PHARMACOLOGYWARNINGS
Exacerbation of symptoms of myasthenia gravis and new onset of symptoms of myasthenic syndrome has been reported in patients receiving erythromycin therapy.
There have been reports of infantile hypertrophic pyloric stenosis (IHPS) occurring in infants following erythromycin therapy. In one cohort of 157 newborns who were given erythromycin for pertussis prophylaxis, seven neonates (5%) developed symptoms of non-bilious vomiting or irritability with feeding and were subsequently diagnosed as having IHPS requiring surgical pyloromyotomy. A possible dose-response effect was described with an absolute risk of IHPS of 5.1% for infants who took erythromycin for 8-14 days and 10% for infants who took erythromycin for 15-21 days. Since erythromycin may be used in the treatment of conditions in infants which are associated with significant mortality or morbidity (such as pertussis or neonatal Chlamydia trachomatis infections), the benefit of erythromycin therapy needs to be weighed against the potential risk of developing IHPS. Parents should be informed to contact their physician if vomiting or irritability with feeding occurs. 5
Prolonged or repeated use of erythromycin may result in an overgrowth of nonsusceptible bacteria or fungi. If superinfection occurs, erythromycin should be discontinued and appropriate therapy instituted.
When indicated, incision and drainage or other surgical procedures should be performed in conjunction with antibiotic therapy.
Observational studies in humans have reported cardiovascular malformations after exposure to drug products containing erythromycin during early pregnancy.
Information for Patients
Patients should be counseled that antibacterial drugs including ERY-TAB should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When ERY-TAB is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by ERY-TAB or other antibacterial drugs in the future. ®®®
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Drug Interactions
Theophylline
Erythromycin use in patients who are receiving high doses of theophylline may be associated with an increase in serum theophylline levels and potential theophylline toxicity. In case of theophylline toxicity and/or elevated serum theophylline levels, the dose of theophylline should be reduced while the patient is receiving concomitant erythromycin therapy.
There have been published reports suggesting that when oral erythromycin is given concurrently with theophylline there is a decrease in erythromycin serum concentrations of approximately 35%. The mechanism by which this interaction occurs is unknown. The decrease in erythromycin concentrations due to co-administration of theophylline could result in subtherapeutic concentrations of erythromycin.
Hypotension, bradyarrhythmias, and lactic acidosis have been observed in patients receiving concurrent verapamil, belonging to the calcium channel blockers drug class.
Concomitant administration of erythromycin and digoxin has been reported to result in elevated digoxin serum levels.
There have been reports of increased anticoagulant effects when erythromycin and oral anticoagulants were used concomitantly. Increased anticoagulation effects due to interactions of erythromycin with oral anticoagulants may be more pronounced in the elderly.
Erythromycin is a substrate and inhibitor of the 3A isoform subfamily of the cytochrome p450 enzyme system (CYP3A). Coadministration of erythromycin and a drug primarily metabolized by CYP3A may be associated with elevations in drug concentrations that could increase or prolong both the therapeutic and adverse effects of the concomitant drug. Dosage adjustments may be considered, and when possible, serum concentrations of drugs primarily metabolized by CYP3A should be monitored closely in patients concurrently receiving erythromycin.
The following are examples of some clinically significant CYP3A based drug interactions. Interactions with other drugs metabolized by the CYP3A isoform are also possible. The following CYP3A based drug interactions have been observed with erythromycin products in post-marketing experience:
Ergotamine/dihydroergotamine
Post-marketing reports indicate that co-administration of erythromycin with ergotamine or dihydroergotamine has been associated with acute ergot toxicity characterized by vasospasm and ischemia of the extremities and other tissues including the central nervous system. Concomitant administration of erythromycin with ergotamine or dihydroergotamine is contraindicated (see ). CONTRAINDICATIONS
Triazolobenzodiazepines (such as triazolam and alprazolam) and related benzodiazepines
Erythromycin has been reported to decrease the clearance of triazolam and midazolam, and thus, may increase the pharmacologic effect of these benzodiazepines.
HMG-CoA Reductase Inhibitors
Erythromycin has been reported to increase concentrations of HMG-CoA reductase inhibitors (e.g., lovastatin and simvastatin). Rare reports of rhabdomyolysis have been reported in patients taking these drugs concomitantly.
Sildenafil (Viagra)
Erythromycin has been reported to increase the systemic exposure (AUC) of sildenafil. Reduction of sildenafil dosage should be considered. (See Viagra package insert.)
There have been spontaneous or published reports of CYP3A based interactions of erythromycin with cyclosporine, carbamazepine, tacrolimus, alfentanil, disopyramide, rifabutin, quinidine, methyl-prednisolone, cilostazol, vinblastine, and bromocriptine.
Concomitant administration of erythromycin with cisapride, pimozide, astemizole, or terfenadine is contraindicated. (See .) CONTRAINDICATIONS
In addition, there have been reports of interactions of erythromycin with drugs not thought to be metabolized by CYP3A, including hexobarbital, phenytoin, and valproate.
Erythromycin has been reported to significantly alter the metabolism of the nonsedating antihistamines terfenadine and astemizole when taken concomitantly. Rare cases of serious cardiovascular adverse events, including electrocardiographic QT/QT interval prolongation, cardiac arrest, torsades de pointes, and other ventricular arrhythmias have been observed. (See .) In addition, deaths have been reported rarely with concomitant administration of terfenadine and erythromycin. cCONTRAINDICATIONS
There have been post-marketing reports of drug interactions when erythromycin was co-administered with cisapride, resulting in QT prolongation, cardiac arrhythmias, ventricular tachycardia, ventricular fibrillation, and torsades de pointes, most likely due to the inhibition of hepatic metabolism of cisapride by erythromycin. Fatalities have been reported. (See .) CONTRAINDICATIONS
Colchicine
Colchicine is a substrate for both CYP3A4 and the efflux transporter P-glycoprotein (P-gp). Erythromycin is considered a moderate inhibitor of CYP3A4. A significant increase in colchicine plasma concentration is anticipated when co-administered with moderate CYP3A4 inhibitors such as erythromycin. If co-administration of colchicine and erythromycin is necessary, the starting dose of colchicine may need to be reduced, and the maximum colchicine dose should be lowered. Patients should be monitored for clinical symptoms of colchicine toxicity (see ). WARNINGS
Drug/Laboratory Test Interactions
Erythromycin interferes with the fluorometric determination of urinary catecholamines.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term oral dietary studies conducted with erythromycin stearate in rats up to 400 mg/kg/day and in mice up to about 500 mg/kg/day (approximately 1-2 fold of the maximum human dose on a body surface area basis) did not provide evidence of tumorigenicity. Erythromycin stearate did not show genotoxic potential in the Ames, and mouse lymphoma assays or induce chromosomal aberrations in CHO cells. There was no apparent effect on male or female fertility in rats treated with erythromycin base by oral gavage at 700 mg/kg/day (approximately 3 times the maximum human dose on a body surface area basis).
Pregnancy
Teratogenic Effects
Pregnancy Category B
There is no evidence of teratogenicity or any other adverse effect on reproduction in female rats fed erythromycin base by oral gavage at 350 mg/kg/day (approximately twice the maximum recommended human dose on a body surface area) prior to and during mating, during gestation, and through weaning. No evidence of teratogenicity or embryotoxicity was observed when erythromycin base was given by oral gavage to pregnant rats and mice at 700 mg/kg/day and to pregnant rabbits at 125 mg/kg/day (approximately 1-3 times the maximum recommended human dose).
Nursing Mothers
Erythromycin is excreted in human milk. Caution should be exercised when erythromycin is administered to a nursing woman.
Geriatric Use
Elderly patients, particularly those with reduced renal or hepatic function, may be at increased risk for developing erythromycin-induced hearing loss. (See and ). ADVERSE REACTIONSDOSAGE AND ADMINISTRATION
Elderly patients may be more susceptible to development of torsades de pointes arrhythmias than younger patients. (See ). WARNINGS
Elderly patients may experience increased effects of oral anticoagulant therapy while undergoing treatment with erythromycin. (See ). PRECAUTIONS - Drug Interactions
Ery-Tab Delayed Release Tablets (250 mg) contain 8.3 mg (0.4 mEq) of sodium per tablet. ®
Ery-Tab Delayed Release Tablets (333 mg) contain 11.2 mg (0.5 mEq) of sodium per tablet. ®
Ery-Tab Delayed Release Tablets (500 mg) contain 16.7 mg (0.7 mEq) of sodium per tablet. ®
The geriatric population may respond with a blunted natriuresis to salt loading. This may be clinically important with regard to such diseases as congestive heart failure.
-
ADVERSE REACTIONS
The most frequent side effects of oral erythromycin preparations are gastrointestinal and are dose-related. They include nausea, vomiting, abdominal pain, diarrhea and anorexia. Symptoms of hepatitis, hepatic dysfunction and/or abnormal liver function test results may occur. (See .) WARNINGS
Onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment. (See .) WARNINGS
Erythromycin has been associated with QT prolongation and ventricular arrhythmias, including ventricular tachycardia and torsades de pointes. (See ). WARNINGS
Allergic reactions ranging from urticaria to anaphylaxis have occurred. Skin reactions ranging from mild eruptions to erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis have been reported rarely.
There have been reports of interstitial nephritis coincident with erythromycin use.
There have been rare reports of pancreatitis and convulsions.
There have been isolated reports of reversible hearing loss occurring chiefly in patients with renal insufficiency and in patients receiving high doses of erythromycin.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
In most patients, ERY-TAB (erythromycin delayed-release tablets) are well absorbed and may be dosed orally without regard to meals. However, optimal blood levels are obtained when ERY-TAB 250 mg, ERY-TAB 333 mg or ERY-TAB 500 mg tablets are given in the fasting state (at least 1/2 hour and preferably 2 hours before meals). ®®®®
Adults
The usual dose is 250 mg four times daily in equally spaced doses. The 333 mg tablet is recommended if dosage is desired every 8 hours. If twice-a-day dosage is desired, the recommended dose is 500 mg every 12 hours. Dosage may be increased up to 4 g per day according to the severity of the infection. However, twice-a-day dosing is not recommended when doses larger than 1 g daily are administered.
Children
Age, weight, and severity of the infection are important factors in determining the proper dosage. The usual dosage is 30 to 50 mg/kg/day, in equally divided doses. For more severe infections, this dose may be doubled but should not exceed 4 g per day.
In the treatment of streptococcal infections of the upper respiratory tract (e.g., tonsillitis or pharyngitis), the therapeutic dosage of erythromycin should be administered for at least ten days.
The American Heart Association suggests a dosage of 250 mg of erythromycin orally, twice a day in long-term prophylaxis of streptococcal upper respiratory tract infections for the prevention of recurring attacks of rheumatic fever in patients allergic to penicillin and sulfonamides. 4
Conjunctivitis of the Newborn Caused by Chlamydia trachomatis
Oral erythromycin suspension 50 mg/kg/day in 4 divided doses for at least 2 weeks. 4
Pneumonia of Infancy Caused by Chlamydia trachomatis
Although the optimal duration of therapy has not been established, the recommended therapy is oral erythromycin suspension 50 mg/kg/day in 4 divided doses for at least 3 weeks.
Urogenital Infections During Pregnancy Due to Chlamydia trachomatis
Although the optimal dose and duration of therapy have not been established, the suggested treatment is 500 mg of erythromycin by mouth four times a day or two erythromycin 333 mg tablets orally every 8 hours on an empty stomach for at least 7 days. For women who cannot tolerate this regimen, a decreased dose of one erythromycin 500 mg tablet orally every 12 hours, one 333 mg tablet orally every 8 hours or 250 mg by mouth four times a day should be used for at least 14 days. 6
For adults with uncomplicated urethral, endocervical, or rectal infections caused by Chlamydia trachomatis, when tetracycline is contraindicated or not tolerated
500 mg of erythromycin by mouth four times a day or two 333 mg tablets orally every 8 hours for at least 7 days. 6
For patients with nongonococcal urethritis caused by Ureaplasma urealyticum when tetracycline is contraindicated or not tolerated
500 mg of erythromycin by mouth four times a day or two 333 mg tablets orally every 8 hours for at least seven days. 6
Acute pelvic inflammatory disease caused by N. gonorrhoeae
500 mg Erythrocin Lactobionate-I.V. (erythromycin lactobionate for injection, USP) every 6 hours for 3 days, followed by 500 mg of erythromycin base orally every 12 hours, or 333 mg of erythromycin base orally every 8 hours for 7 days.
Pertussis
Although optimal dosage and duration have not been established, doses of erythromycin utilized in reported clinical studies were 40 to 50 mg/kg/day, given in divided doses for 5 to 14 days.
Legionnaires' Disease
Although optimal dosage has not been established, doses utilized in reported clinical data were 1 to 4 g daily in divided doses.
Preoperative Prophylaxis for Elective Colorectal Surgery
Listed below is an example of a recommended bowel preparation regimen.
A proposed surgery time of 8:00 a.m. has been used.
Pre-op Day 2
Minimum residue or clear liquid diet. Magnesium sulfate, 30 mL, 50% solution (15 g) orally at 10:00 a.m., 2:00 p.m. and 6:00 p.m. Enema at 7:00 p.m. and 8:00 p.m.
Pre-op Day 1
Clear liquid diet. Supplemental (IV) fluids as needed. Magnesium sulfate, 30 mL, 50% solution (15 g) orally at 10:00 a.m. and 2:00 p.m. Neomycin sulfate (1.0 g) and erythromycin base (two 500 mg tablets, three 333 mg tablets or four 250 mg tablets) orally at 1:00 p.m., 2:00 p.m. and 11:00 p.m. No enema.
-
HOW SUPPLIED
NDC: 68151-1170-1 in a PACKAGE of 1 TABLET, DELAYED RELEASES
-
REFERENCES
- Clinical and Laboratory Standards Institute (CLSI). CLSI document M07-A9, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2012. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard – Ninth Edition.
- Clinical and Laboratory Standards Institute (CLSI). CLSI document M100-S23, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2013. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-third Informational Supplement.
- Clinical and Laboratory Standards Institute (CLSI). CLSI document M02-A11. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2012. Performance Standards for Antimicrobial Disk Diffusion Susceptibility Tests; Approved Standard – Eleventh Edition
- Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, the American Heart Association: Prevention of Rheumatic Fever. Circulation. 78(4):1082-1086, October 1988.
- Honein, M.A., et. al.: Infantile hypertrophic pyloric stenosis after pertussis prophylaxis with erythromycin: a case review and cohort study. The Lancet 1999:354 (9196): 2101-5.
- Data on file, Arbor Pharmaceuticals, LLC.
- SPL UNCLASSIFIED SECTION
- Erythromycin
-
INGREDIENTS AND APPEARANCE
ERY-TAB
erythromycin tablet, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68151-1170(NDC:24338-122) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Erythromycin (UNII: 63937KV33D) (Erythromycin - UNII:63937KV33D) Erythromycin 250 mg Inactive Ingredients Ingredient Name Strength AMMONIA (UNII: 5138Q19F1X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CROSPOVIDONE (UNII: 68401960MK) DIACETYLATED MONOGLYCERIDES (UNII: 5Z17386USF) HYDROXYPROPYL CELLULOSE (TYPE H) (UNII: RFW2ET671P) HYPROMELLOSES (UNII: 3NXW29V3WO) HYPROMELLOSE PHTHALATE (24% PHTHALATE, 55 CST) (UNII: 87Y6436BKR) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONES (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM CITRATE (UNII: 1Q73Q2JULR) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape OVAL Size 15mm Flavor Imprint Code A;EC Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68151-1170-1 1 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA062298 04/18/2011 Labeler - Carilion Materials Management (079239644) Registrant - Carilion Materials Management (079239644) Establishment Name Address ID/FEI Business Operations Carilion Materials Management 079239644 REPACK(68151-1170)
Trademark Results [ERY-TAB]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ERY-TAB 73286993 1211217 Live/Registered |
Abbott Laboratories 1980-11-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
