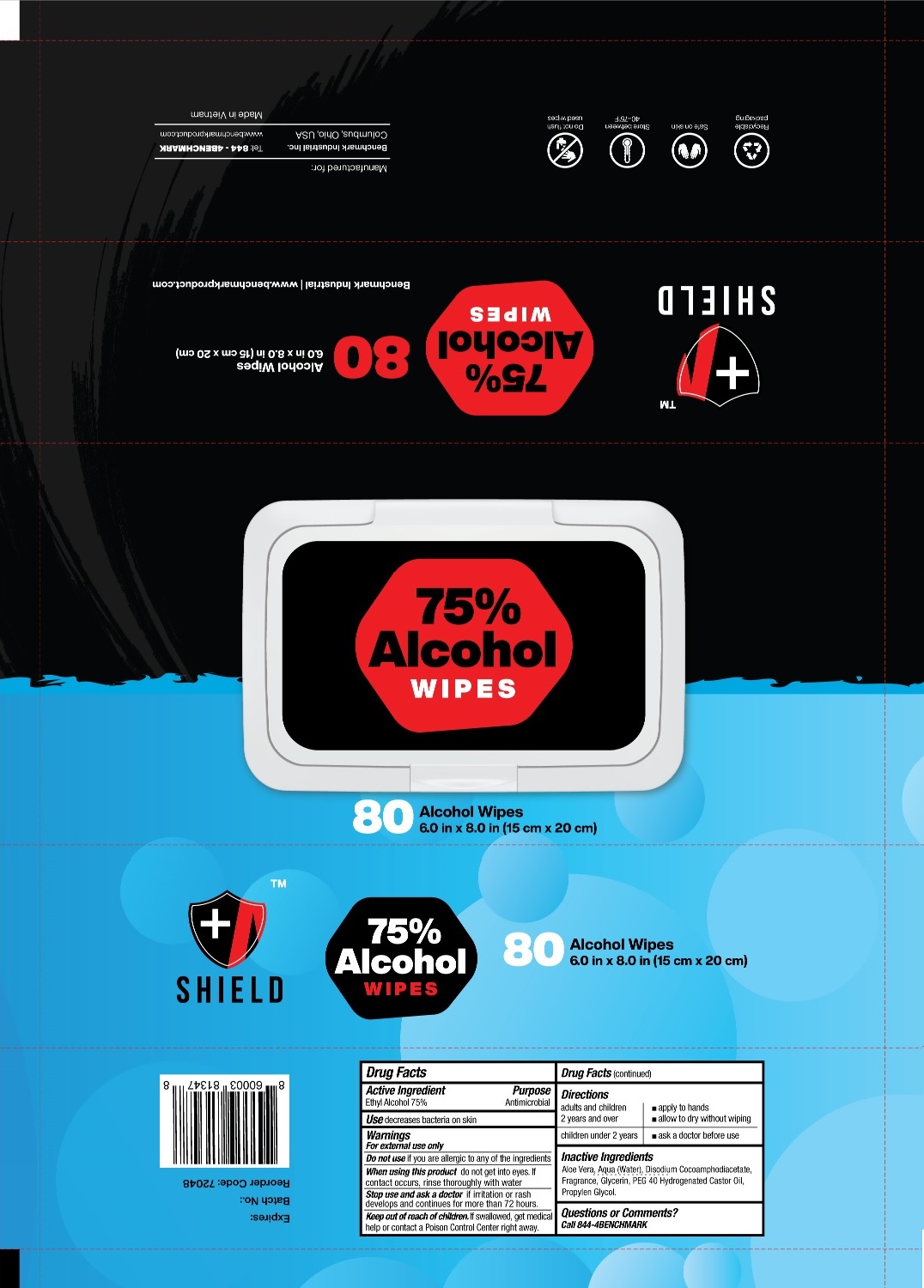
Shield by Benchmark Industrial Inc. SHIELD 75% Alcohol WIPES
Shield by
Drug Labeling and Warnings
Shield by is a Otc medication manufactured, distributed, or labeled by Benchmark Industrial Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SHIELD- ethyl alcohol cloth
Benchmark Industrial Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
SHIELD 75% Alcohol WIPES
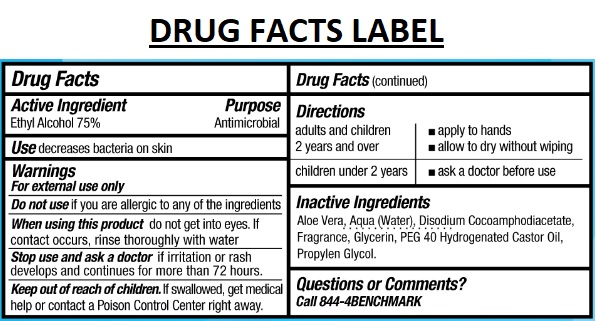
Warnings
For external use only
Do not use if you are allergic to any of the ingredients
When using this product do not get into eyes. If contact occurs, rinse thoroughly with water
Stop use and ask a doctor if irritation or rash develops and continues for more than 72 hours.
Directions
| adults and children 2 years and over | apply to hands allow to dry without wiping |
| children under 2 years | ask a doctor before use |
Inactive Ingredients
Aloe Vera, Aqua (Water), Disodium Cocoamphodiacetate, Fragrance, Glycerin, PEG 40 Hydrogenated Castor Oil, Propylene Glycol.
| SHIELD
ethyl alcohol cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Benchmark Industrial Inc. (004284782) |
Trademark Results [Shield]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SHIELD 98853970 not registered Live/Pending |
AUTOMATED SYSTEMS OF TACOMA, LLC 2024-11-14 |
 SHIELD 98774983 not registered Live/Pending |
Kemin Industries, Inc. 2024-09-27 |
 SHIELD 98504292 not registered Live/Pending |
CHANNELL COMMERCIAL CORPORATION 2024-04-17 |
 SHIELD 98403858 not registered Live/Pending |
The Toro Company 2024-02-13 |
 SHIELD 98269465 not registered Live/Pending |
Shield Skin, LLC 2023-11-14 |
 SHIELD 98232915 not registered Live/Pending |
SuperBam, Inc. 2023-10-20 |
 SHIELD 98143079 not registered Live/Pending |
SHIELD 2023-08-21 |
 SHIELD 98143079 not registered Live/Pending |
BlackStar Battlefield Solutions 2023-08-21 |
 SHIELD 98137632 not registered Live/Pending |
Filters Delivered L.L.C. 2023-08-17 |
 SHIELD 98075627 not registered Live/Pending |
Grussmark, Stephen M. 2023-07-07 |
 SHIELD 97923412 not registered Live/Pending |
SANWA USA HOLDINGS, INC. 2023-05-05 |
 SHIELD 97889188 not registered Live/Pending |
Inkit Inc. 2023-04-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.