ZYLOTROL PAIN RELIEVING CREAM
ZYLOTROL PAIN RELIEVING by
Drug Labeling and Warnings
ZYLOTROL PAIN RELIEVING by is a Otc medication manufactured, distributed, or labeled by Whitestone Products LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ZYLOTROL PAIN RELIEVING- lidocaine hcl, menthol cream
Whitestone Products LLC
----------
ZYLOTROL PAIN RELIEVING CREAM
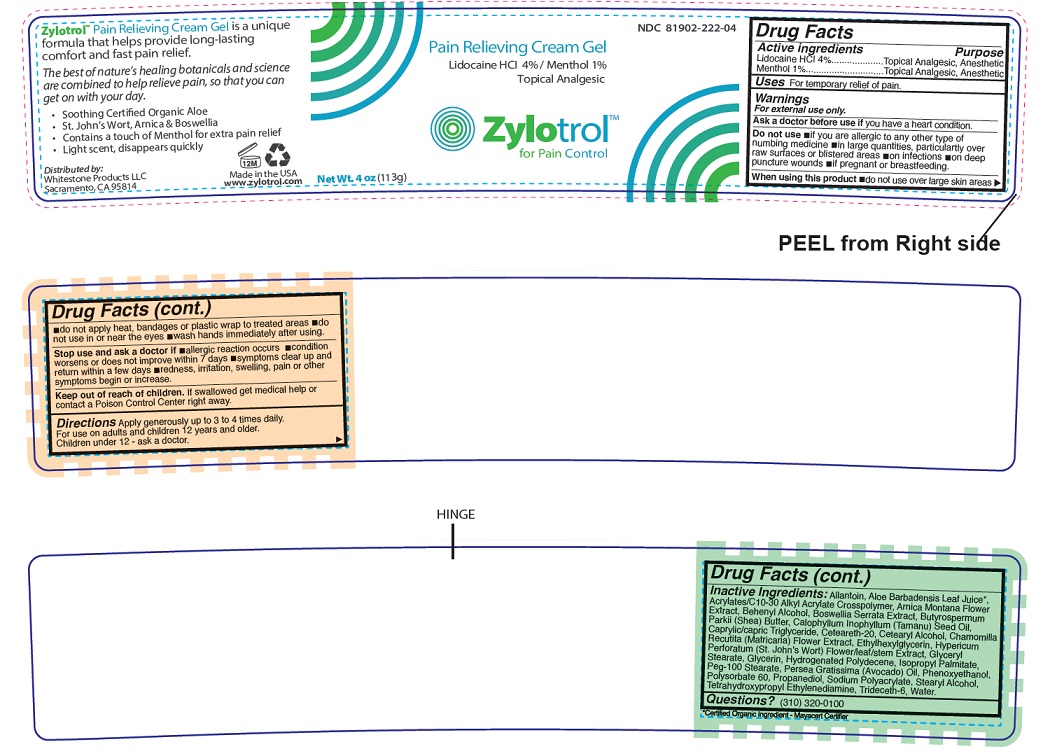
Warnings
For external use only.
Ask a doctor before use if you have a heart condition.
Do not use if you are allergic to any other type of numbing medicine in large quantities, particularly over raw surfaces or blistered areas on infections on deep puncture wounds if pregnant or breastfeeding.
When using this product do not use over large skin areas do not apply heat, bandages or plastic wrap to treated areas do not use in or near the eyes wash hands immediately after using.
Stop use and ask a doctor if allergic reaction occurs condition worsens or does not improve within 7 days symptoms clear up and return within a few days redness, irritation, swelling, pain or other symptoms begin or increase.
Directions
Apply generously up to 3 to 4 times daily. For use on adults and children 12 years and older. Children under 12 - ask a doctor.
Inactive ingredients:
Allantoin, Aloe Barbadensis Leaf Juice*, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Arnica Montana Flower Extract, Behenyl Alcohol, Boswellia Serrata Extract, Butyrospermum Parkii (Shea) Butter, Calophyllum Inophyllum (Tamanu) Seed Oil, Caprylic/capric Triglyceride, Ceteareth-20, Cetearyl Alcohol, Chamomilla Recutita (Matricaria) Flower Extract, Ethylhexylglycerin, Hypericum Perforatum (St. John's Wort) Flower/leaf/stem Extract, Glyceryl Stearate, Glycerin, Hydrogenated Polydecene, Isopropyl Palmitate, Peg-100 Stearate, Persea Gratissima (Avocado) Oil, Phenoxyethanol, Polysorbate 60, Propanediol, Sodium Polyacrylate, Stearyl Alcohol, Tetrahydroxypropyl Ethylenediamine, Trideceth-6, Water.
*Certified Organic Ingredient - Mayacert Certifier
| ZYLOTROL PAIN RELIEVING
lidocaine hcl, menthol cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Whitestone Products LLC (118064415) |