Carring Mill Itch Relief Pump Spray
Itch Relief by
Drug Labeling and Warnings
Itch Relief by is a Otc medication manufactured, distributed, or labeled by FSA Store Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ITCH RELIEF- diphenhydramine hcl and zinc acetate spray
FSA Store Inc.
----------
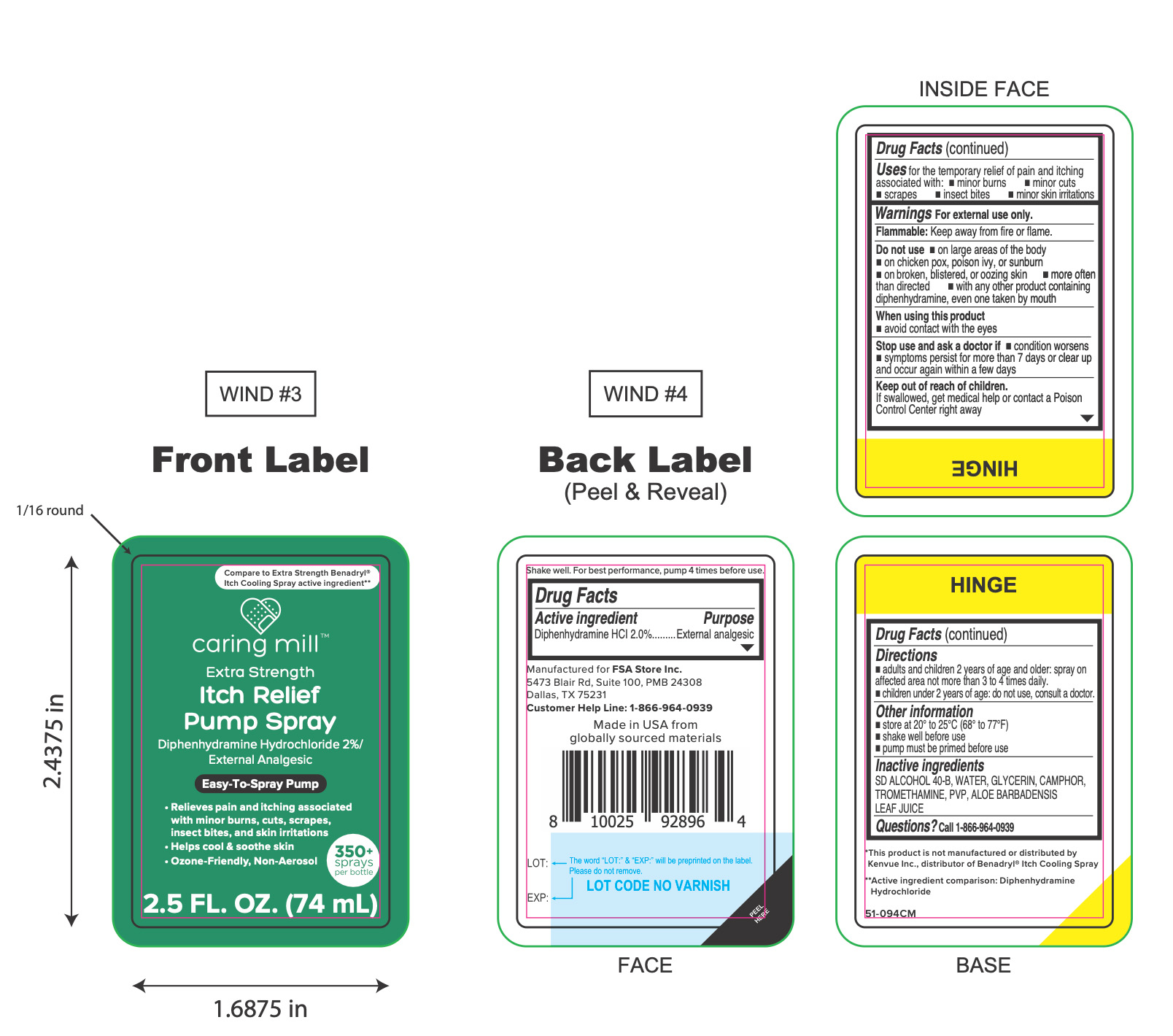
Carring Mill Itch Relief Pump Spray
Uses
for the temporary relief of pain and itching associated with:
- minor burns
- minor cuts
- scrapes
- insect bites
- minor skin irritations
Warnings
For external use only.
Do not use
- on large areas of the body
- on chicken pox, poison ivy, or sunburn
- on broken, blistered, or oozing skin
- more often than directed
- with any other product containing diphenhydramine, even one taken by mouth
Directions
- adults and children 2 years of age and older: spray on affected area not more than 3 to 4 times daily.
- children under 2 years of age: do not use, consult a doctor.
Other information
- store between 20° to 25°C (68° to 77°F)
- shake well before use
- pump must be primed before use
Inactive ingredients
SD ALCOHOL 40-B, WATER, GLYCERIN, CAMPHOR, TROMETHAMINE, PVP, ALOE BARBADENSIS LEAF JUICE
Principal Display Panel
caring mill
Extra Strength
Itch Relief
Pump Spray
Diphenhydramine Hydrochloride 2%/
External Analgesic
Easy-To-Spray Pump
- Relieves pain and itching associated
with minor burns, cuts, scrapes,
insect bites, and skin irritations
- Helps cool & soothe skin
- Ozone-Friendly, Non-Aerosol
2.5 FL. OZ. (74 mL)
| ITCH RELIEF
diphenhydramine hcl and zinc acetate spray |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - FSA Store Inc. (049283340) |
Revised: 12/2025
Document Id: 47436dfc-2331-fde3-e063-6294a90a0afb
Set id: 3cd1933c-6341-50d6-e063-6294a90aca32
Version: 2
Effective Time: 20251231
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.