AVACLYR- acyclovir ointment
Avaclyr by
Drug Labeling and Warnings
Avaclyr by is a Prescription medication manufactured, distributed, or labeled by Fera Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AVACLYR safely and effectively. See full prescribing information for AVACLYR.
AVACLYR® (acyclovir ophthalmic ointment) 3%, for topical ophthalmic use
Initial U.S. Approval: 1982INDICATIONS AND USAGE
AVACLYR (acyclovir ophthalmic ointment) 3%, a herpes simplex virus nucleoside analog DNA polymerase inhibitor, is indicated in the treatment of acute herpetic keratitis (dendritic ulcers) in patients with herpes simplex (HSV-1 and HSV-2) virus. (1)
DOSAGE AND ADMINISTRATION
Apply a 1 cm ribbon in the lower cul-de-sac of the affected eye 5 times per day until healed then 3 times per day for 7 days. (2)
DOSAGE FORMS AND STRENGTHS
Ophthalmic ointment containing 3% acyclovir. (3)
CONTRAINDICATIONS
AVACLYR is contraindicated in patients with a known hypersensitivity to acyclovir or valacyclovir. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse reactions (2 to 10%) reported in patients were eye pain (stinging), punctate keratitis and follicular conjunctivitis. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Fera Pharmaceuticals, LLC at (414) 434-6604 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Topical Ophthalmic Use
5.2 Avoidance of Contact Lenses
5.3 Risk of Contamination
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NON-CLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.2 Avoidance of Contact Lenses
Patients should not wear contact lenses if they have signs or symptoms of herpetic keratitis or during the course of therapy with AVACLYR.
5.3 Risk of Contamination
This product is sterile when packaged. Patients should be advised to not allow the tip of the container to touch any surface, as this may contaminate the ointment. If pain develops, or if redness, itching, or inflammation becomes aggravated, the patient should be advised to consult a physician.
-
6 ADVERSE REACTIONS
The most common adverse reactions (2-10%) reported in patients were eye pain (stinging), punctate keratitis and follicular conjunctivitis. Rare reports of blepharitis and very rare reports of immediate hypersensitivity reactions including angioedema and urticaria have been observed post-marketing in patients taking AVACLYR.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
A prospective epidemiologic registry of acyclovir use from 1984 to 1999 indicated that the occurrence rate of birth defects in women exposed to systemically administered acyclovir during the first trimester of pregnancy (period of organogenesis) approximated that found in the general population. Likewise, oral and subcutaneous administration of acyclovir to pregnant mice, rats, and rabbits during organogenesis did not produce teratogenicity at clinically relevant doses (see Animal Data).
Data
Human Data
A prospective epidemiologic registry of acyclovir use during pregnancy was established in 1984 and completed in April 1999. There were 749 pregnancies followed in women exposed to systemically administered acyclovir during the first trimester of pregnancy resulting in 756 outcomes. The occurrence rate of birth defects approximates that found in the general population. However, the small size of the registry is insufficient to evaluate the risk for less common defects, or to permit reliable or definitive conclusions regarding the safety of acyclovir in pregnant women and their developing fetuses. The human maternal plasma level of acyclovir following ocular administration is unknown.
Animal Data
In published animal reproduction studies, acyclovir was not maternally toxic and did not produce teratogenicity in the mouse at oral doses up to 450 mg/kg/day (1100 times the maximum recommended human ophthalmic dose [RHOD] on a mg/m2 basis, assuming 100% absorption), or in the rat and rabbit at subcutaneous doses up to 50 mg/kg/day (approximately 250 and 500 times the RHOD, respectively) when administered throughout the period of organogenesis.
Administration of acyclovir from postnatal days 3 to 21 did not produce adverse effects in neonatal rats at subcutaneous doses less than or equal to 20 mg/kg/day (100 times the RHOD).
8.2 Lactation
Risk Summary
Acyclovir concentrations have been documented in breast milk following oral administration of acyclovir. There is no information regarding the presence of acyclovir in human milk following ocular administration, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for acyclovir, and any potential adverse effects on the breast-fed child from acyclovir or from the underlying maternal condition.
- 10 OVERDOSAGE
-
11 DESCRIPTION
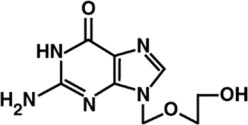
Acyclovir is a synthetic herpes simplex virus nucleoside analog DNA polymerase inhibitor. The drug substance is a white crystalline powder with the molecular formula of C8H11N5O3 and a molecular weight of 225.2. The maximum solubility in water at 25°C is 1.41 mg/mL. The pka's of acyclovir are 2.52 and 9.35.
The chemical name of acyclovir is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]6H-purin-6-one, it has the following chemical structure:
AVACLYR is a sterile ointment for topical administration in eyes. Each gram of ointment contains 30 mg of acyclovir in a white petrolatum base.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
It has not been possible to detect acyclovir concentrations in the blood by existing bioanalytical methods after topical application to the eye. Trace quantities are detectable in the urine but are not therapeutically relevant.
12.4 Microbiology
Acyclovir is a synthetic purine nucleoside analogue that is phosphorylated intracellularly by the viral encoded thymidine kinase (TK) of HSV into acyclovir monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In a biochemical reaction, acyclovir triphosphate inhibits replication of herpes viral DNA by competing with nucleotides for binding to the viral DNA polymerase and by incorporation into and termination of the growing viral DNA chain. The cellular thymidine kinase of normal, uninfected cells does not use acyclovir effectively as a substrate, hence toxicity to mammalian host cells is low.
Antiviral Activities: The quantitative relationship between the cell culture susceptibility of herpes virus to antivirals and the clinical response to therapy has not been established in humans, and virus sensitivity testing has not been standardized. Sensitivity testing results, expressed as the concentration of drug required to inhibit by 50% the growth of virus in cell culture (EC50), vary greatly depending upon a number of factors. Using plaque-reduction assays, on Vero cells, the median EC50 value of acyclovir against clinical herpes simplex virus isolates (subjects receiving placebo) was 1.3 μM (range: < 0.56 to 3.3 μM).
Drug Resistance: Resistance of HSV to acyclovir can result from qualitative and quantitative changes in the viral TK and/or DNA polymerase. Clinical isolates of HSV with reduced susceptibility to acyclovir have been recovered from immunocompromised patients, especially with advanced HIV infection. While most of the acyclovir resistant mutant isolates thus far from such patients have been found to be TK deficient, other mutant isolates involving the viral TK gene (TK partial and TK altered) or DNA polymerase have been identified. TK negative mutants may cause severe disease in infants and immunocompromised adults. The possibility of viral resistance to acyclovir should be considered in immunocompromised patients who show poor clinical response during therapy.
-
13 NON-CLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Acyclovir was not shown to be carcinogenic in mouse and rat bioassays at oral doses up to 450 mg/kg (approximately 1100 – 2200 times the maximum RHOD, on a mg/m2 basis, assuming 100% absorption).
Mutagenesis
Acyclovir was tested in 16 in vitro and in vivo genetic toxicity assays. Acyclovir was found to be negative in the Ames test, positive in the in vitro mouse lymphoma assay (TK locus), and positive in the in vitro and in vivo assays for chromosomal effects.
Impairment of Fertility
In reproduction studies, acyclovir did not impair fertility or reproduction at oral doses up to 450 mg/kg/day in mice (1100 times the RHOD), or at subcutaneous doses of 25 mg/kg/day in rats (125 times the RHOD). At a dose of 50 mg/kg/day in rats and rabbits (250 and 500 times the RHOD, respectively), implantation efficiency was decreased.
-
14 CLINICAL STUDIES
In five randomized, double masked studies which enrolled a total of 238 subjects with dendritic herpetic keratitis, acyclovir ophthalmic ointment, 3% was either superior or as effective as idoxuridine ophthalmic ointment 0.5% or 1% in subjects with dendritic ulcers. Clinical resolution (healed ulcers) at Day 7 averaged 83% for acyclovir and 50% for idoxuridine.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
AVACLYR is available in a 3.5 g aluminum tube with a white high density polyethylene cap as a clear, colorless, sterile ophthalmic ointment for topical use containing 3% acyclovir active drug. Each tube is packaged in an individual carton.
3.5 g tube (NDC: 48102-028-35)
Store at 15°C to 25°C (59°F to 77°F).
-
17 PATIENT COUNSELING INFORMATION
Administration
Advise patients to wash hands well and pull down lower lid of the affected eye to form a pocket. Instruct the patient to apply a 1 cm (1/2 inch) ribbon of ointment in the pocket formed by the lower lid 5 times per day (approximately every 3 hours while awake). After application of the ointment, the patient should be advised to close their eye for 1-2 minutes. Excess ointment can be wiped away. Continue this dosing 5 times per day until the patient is advised by their physician that the corneal ulcer is healed. Once healed, instruct the patient to continue use of a 1 cm (1/2 inch) ribbon of ointment 3 times per day for 7 more days. [see Dosage and Administration (2)].
Risk of Contamination
This product is sterile when packaged. Advise patients to not allow the tip of the container to touch any surface, as this may contaminate the ointment.
-
Principal Display Panel - 30 mg Carton Label
FERA
NDC: 48102-028-35
Avaclyr®
(Acyclovir
Ophthalmic
Ointment) 3%For
Topical
Ophthalmic
Use.STERILE
3.5g Net Wt. Rx only
-
Principal Display Panel - 30 mg Tube Label
FERA
NDC: 48102-028-35
Avaclyr®
(Acyclovir
Ophthalmic
Ointment) 3%Each gram contains:
acyclovir USP 30 mg
in an ointment base
of white petrolatum.For Topical
Ophthalmic Use.Sterile
3.5g Net Wt. Rx only
Lot:
-
INGREDIENTS AND APPEARANCE
AVACLYR
acyclovir ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 48102-028 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACYCLOVIR (UNII: X4HES1O11F) (ACYCLOVIR - UNII:X4HES1O11F) ACYCLOVIR 30 mg in 1 g Inactive Ingredients Ingredient Name Strength WHITE PETROLATUM (UNII: B6E5W8RQJ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 48102-028-35 1 in 1 CARTON 01/31/2026 1 3.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202408 01/31/2026 Labeler - Fera Pharmaceuticals, LLC (831023713)
Trademark Results [Avaclyr]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AVACLYR 87448101 not registered Live/Pending |
Pragma Pharmaceuticals 2017-05-12 |
 AVACLYR 86063674 not registered Dead/Abandoned |
Pragma 2013-09-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.