DOCUSATE SODIUM AND SENNOSIDES tablet
Docusate Sodium and Sennosides by
Drug Labeling and Warnings
Docusate Sodium and Sennosides by is a Otc medication manufactured, distributed, or labeled by Morepen Laboratories Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
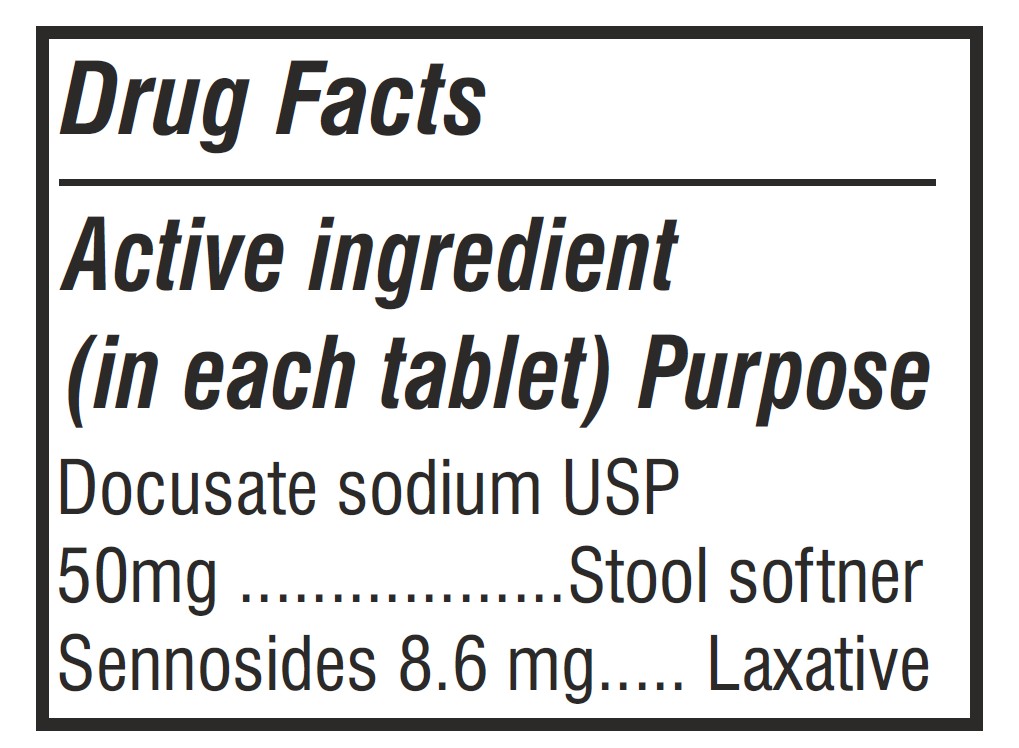
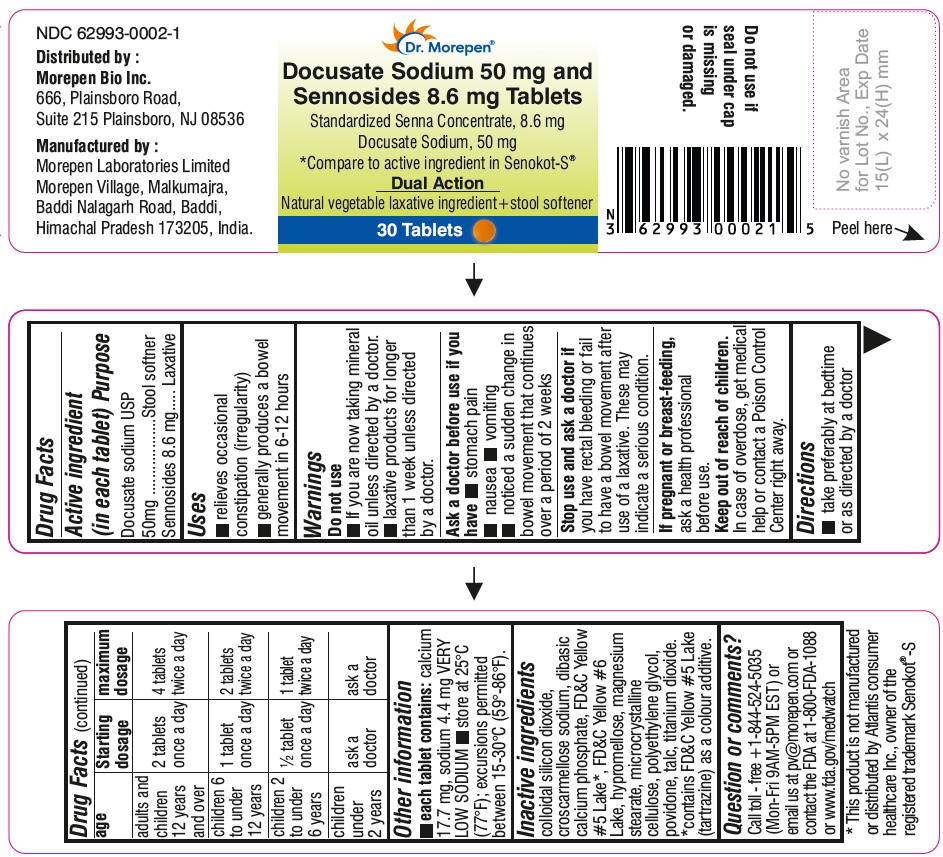
- Active Ingredients Label
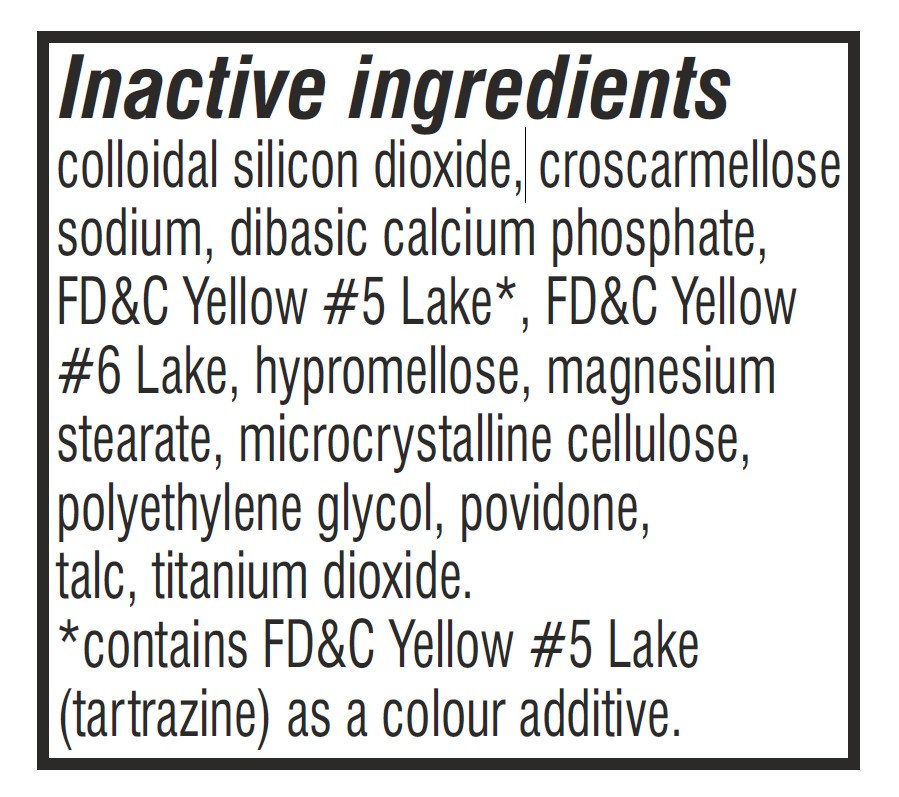
- Inactive Ingredients Section Label
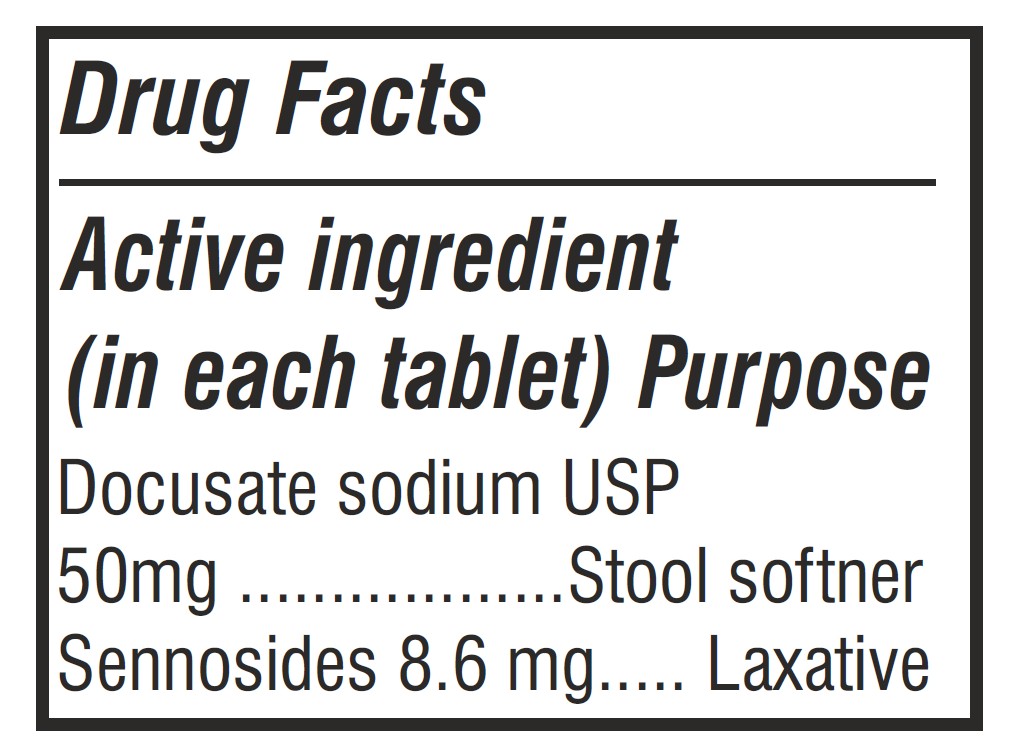
- Purpose Section Label
- Indications & Usage Seciton Label
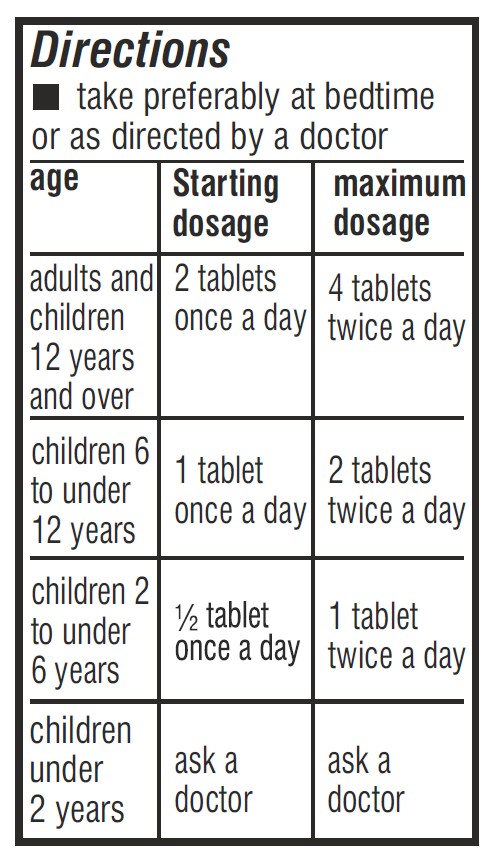
- Dosage & Administration Section Label
- Questions Section Label
- Warnings Section Label
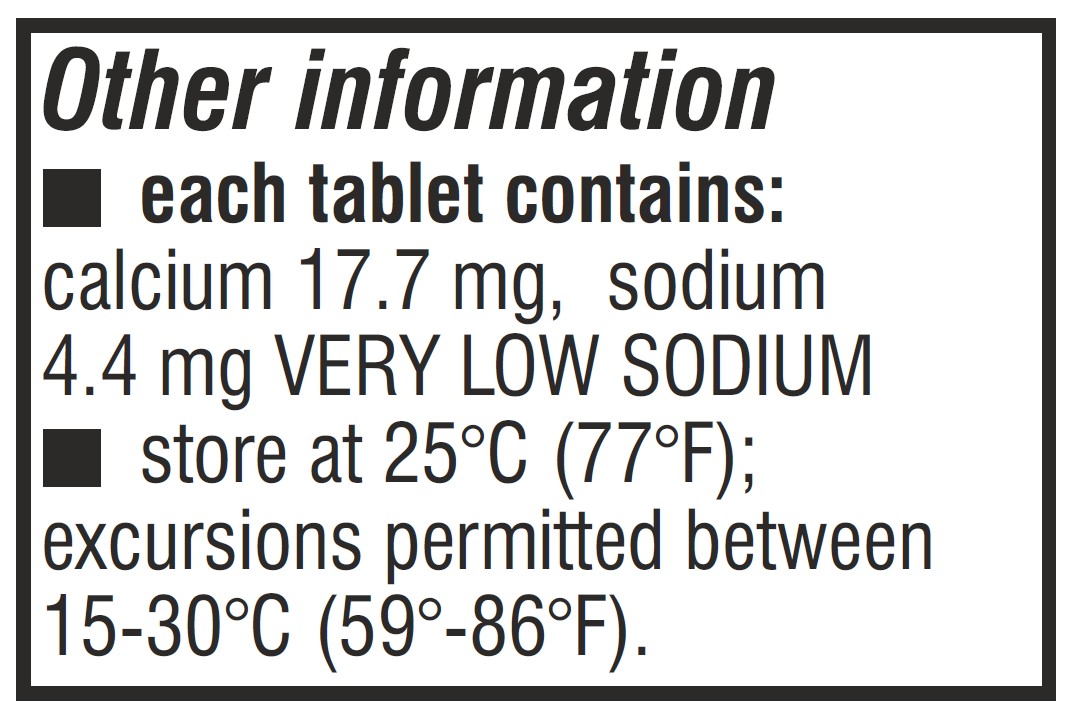
- Storage and Handling Section Label
- 30 Tablets Bottle Label (NDC: 62993-0002-1)
- 60 Tablets Bottle Label (NDC: 62993-0002-2)
- 100 Tablets Bottle Label (NDC: 62993-0002-3)
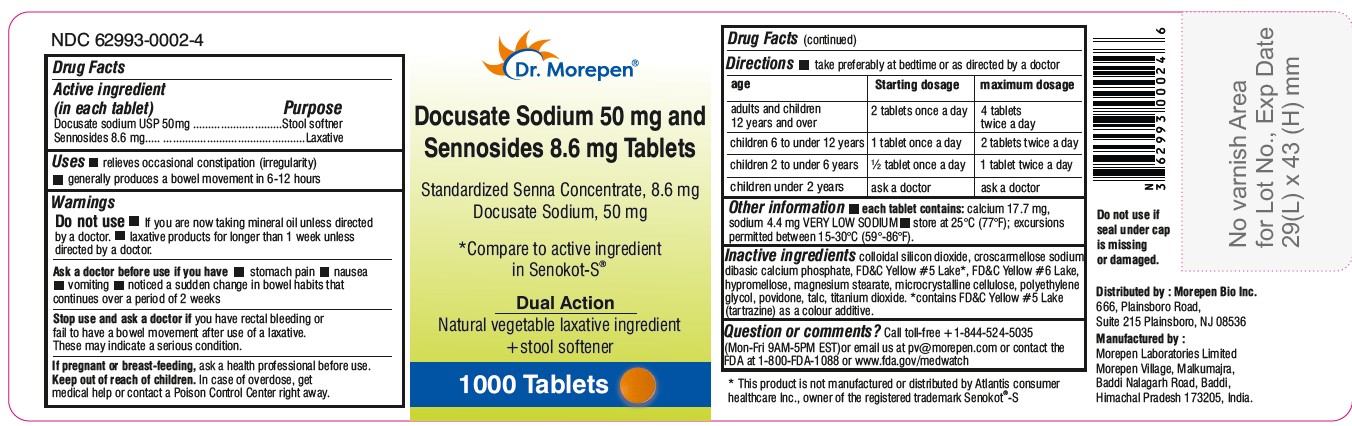
- 1000 Tablets Bottle Label (NDC: 62993-0002-4)
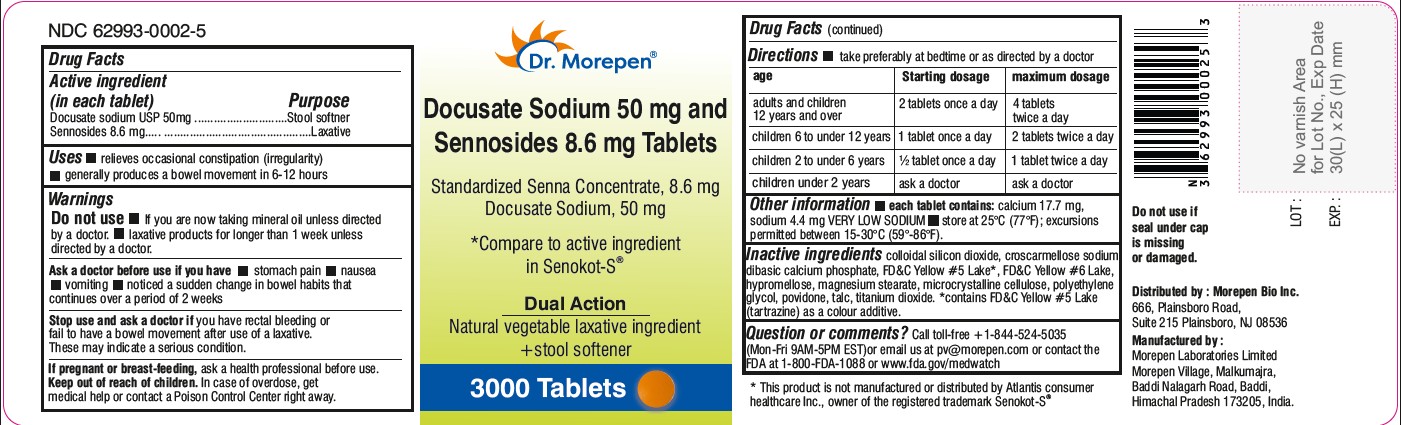
- 3000 Tablets Bottle Label (NDC: 62993-0002-5)
-
INGREDIENTS AND APPEARANCE
DOCUSATE SODIUM AND SENNOSIDES
docusate sodium and sennosides tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62993-0002 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) TALC (UNII: 7SEV7J4R1U) POVIDONE K30 (UNII: U725QWY32X) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Product Characteristics Color orange Score no score Shape ROUND Size 10mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62993-0002-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2025 2 NDC: 62993-0002-2 60 in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2025 3 NDC: 62993-0002-3 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2025 4 NDC: 62993-0002-4 1000 in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2025 5 NDC: 62993-0002-5 3000 in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 10/01/2025 Labeler - Morepen Laboratories Limited (650087067) Registrant - Morepen Laboratories Limited (772653251) Establishment Name Address ID/FEI Business Operations Morepen Laboratories Limited 772653251 manufacture(62993-0002) , pack(62993-0002) , analysis(62993-0002) , label(62993-0002)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.