Mesalamine by ANI Pharmaceuticals, Inc. MESALAMINE enema
Mesalamine by
Drug Labeling and Warnings
Mesalamine by is a Prescription medication manufactured, distributed, or labeled by ANI Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
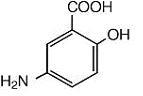
The active ingredient in Mesalamine Rectal Suspension Enema, a disposable (60 mL) unit, is mesalamine, also known as 5-aminosalicylic acid (5-ASA). Chemically, mesalamine is 5-amino-2-hydroxybenzoic acid.
The empirical formula is C7H7NO3, representing a molecular weight of 153.14. The structural formula is:
Each rectal suspension enema unit contains 4 grams of mesalamine. In addition to mesalamine the preparation contains the inactive ingredients carbomer 934P, edetate disodium, potassium acetate, potassium metabisulfite, purified water and xanthan gum. Sodium benzoate is added as a preservative. The disposable unit consists of an applicator tip protected by a polyethylene cover and lubricated with USP white petrolatum. The unit has a one-way valve to prevent back flow of the dispensed product.
-
CLINICAL PHARMACOLOGY
Sulfasalazine is split by bacterial action in the colon into sulfapyridine (SP) and mesalamine (5-ASA). It is thought that the mesalamine component is therapeutically active in ulcerative colitis [A.K. Azad Khan et al, Lancet 2:892-895 (1977)]. The usual oral dose of sulfasalazine for active ulcerative colitis in adults is two to four grams per day in divided doses. Four grams of sulfasalazine provide 1.6 g of free mesalamine to the colon. Each Mesalamine Rectal Suspension Enema delivers up to 4 g of mesalamine to the left side of the colon.
The mechanism of action of mesalamine (and sulfasalazine) is unknown, but appears to be topical rather than systemic. Mucosal production of arachidonic acid (AA) metabolites, both through the cyclooxygenase pathways, i.e., prostanoids, and through the lipoxygenase pathways, i.e., leukotrienes (LTs) and hydroxyeicosatetraenoic acids (HETEs) is increased in patients with chronic inflammatory bowel disease, and it is possible that mesalamine diminishes inflammation by blocking cyclooxygenase and inhibiting prostaglandin (PG) production in the colon.
Preclinical Toxicology
Preclinical studies have shown the kidney to be the major target organ for mesalamine toxicity. Adverse renal function changes were observed in rats after a single 600 mg/kg oral dose, but not after a 200 mg/kg dose. Gross kidney lesions, including papillary necrosis, were observed after a single oral >900 mg/kg dose, and after I.V. doses of >214 mg/kg. Mice responded similarly. In a 13-week oral (gavage) dose study in rats, the high dose of 640 mg/kg/day mesalamine caused deaths, probably due to renal failure, and dose-related renal lesions (papillary necrosis and/or multifocal tubular injury) were seen in most rats given the high dose (males and females) as well as in males receiving lower doses 160 mg/kg/day. Renal lesions were not observed in the 160 mg/kg/day female rats. Minimal tubular epithelial damage was seen in the 40 mg/kg/day males and was reversible. In a six-month oral study in dogs, the no-observable dose level of mesalamine was 40 mg/kg/day and doses of 80 mg/kg/day and higher caused renal pathology similar to that described for the rat. In a combined 52-week toxicity and 127-week carcinogenicity study in rats, degeneration in kidneys was observed at doses of 100 mg/kg/day and above admixed with diet for 52 weeks, and at 127 weeks increased incidence of kidney degeneration and hyalinization of basement membranes and Bowman’s capsule were seen at 100 mg/kg/day and above. In the 12-month eye toxicity study in dogs, Keratoconjunctivitis Sicca (KCS) occurred at oral doses of 40 mg/kg/day and above. The oral preclinical studies were done with a highly bioavailable suspension where absorption throughout the gastrointestinal tract occurred. The human dose of 4 grams represents approximately 80 mg/kg but when mesalamine is given rectally as a suspension, absorption is poor and limited to the distal colon (see Pharmacokinetics). Overt renal toxicity has not been observed (see ADVERSE REACTIONS and PRECAUTIONS), but the potential must be considered.
Pharmacokinetics
Mesalamine administered rectally as Mesalamine Rectal Suspension Enema is poorly absorbed from the colon and is excreted principally in the feces during subsequent bowel movements. The extent of absorption is dependent upon the retention time of the drug product, and there is considerable individual variation. At steady state, approximately 10 to 30% of the daily 4-gram dose can be recovered in cumulative 24-hour urine collections. Other than the kidney, the organ distribution and other bioavailability characteristics of absorbed mesalamine in man are not known. It is known that the compound undergoes acetylation but whether this process takes place at colonic or systemic sites has not been elucidated.
Whatever the metabolic site, most of the absorbed mesalamine is excreted in the urine as the N-acetyl-5-ASA metabolite. The poor colonic absorption of rectally administered mesalamine is substantiated by the low serum concentration of 5-ASA and N-acetyl-5-ASA seen in ulcerative colitis patients after dosage with mesalamine. Under clinical conditions patients demonstrated plasma levels 10 to 12 hours post mesalamine administration of 2 μg/mL, about two-thirds of which was the N-acetyl metabolite. While the elimination half-life of mesalamine is short (0.5 to 1.5 h), the acetylated metabolite exhibits a half-life of 5 to 10 hours [U. Klotz, Clin. Pharmacokin. 10:285-302 (1985)]. In addition, steady state plasma levels demonstrated a lack of accumulation of either free or metabolized drug during repeated daily administrations.
Efficacy
In a placebo-controlled, international, multicenter trial of 153 patients with active distal ulcerative colitis, proctosigmoiditis or proctitis, Mesalamine Rectal Suspension Enema reduced the overall disease activity index (DAI) and individual components as follows:
EFFECT OF TREATMENT ON SEVERITY OF DISEASE DATA FROM U.S.-CANADA TRIAL COMBINED RESULTS OF EIGHT CENTERS
Activity Indices, MeanN Baseline Day
22End
PointChange Baseline to End point* Overall DAI
Mesalamine Rectal Suspension Enema
76
7.42
4.05†
3.37‡
-55.07%‡
Placebo
77
7.40
6.03
5.83
-21.58%
Stool Frequency
Mesalamine Rectal Suspension Enema
1.58
1.11§
1.01†
-0.57§
Placebo
1.92
1.47
1.50
-0.41
Rectal Bleeding
Mesalamine Rectal Suspension Enema
1.82
0.59‡
0.51‡
-1.30‡
Placebo
1.73
1.21
1.11
-0.61
Mucosal Inflammation
Mesalamine Rectal Suspension Enema
2.17
1.22†
0.96‡
-1.21†
Placebo
2.18
1.74
1.61
-0.56
Physician's Assessment of
Mesalamine Rectal Suspension Enema
1.86
1.13‡
0.88‡
-0.97‡
Disease Severity
Placebo
1.87
1.62
1.55
-0.30
Each parameter has a 4-point scale with a numerical rating:
0 = normal, 1 = mild, 2 = moderate, 3 = severe. The four parameters are added together to produce a maximum overall DAI of 12.-
* Percent change for overall DAI only (calculated by taking the average of the change for each individual patient).
† Significant Mesalamine Rectal Suspension Enema/placebo difference. p < 0.01
‡ Significant Mesalamine Rectal Suspension Enema/placebo difference. p < 0.001
§ Significant Mesalamine Rectal Suspension Enema/placebo difference. p < 0.05
Differences between Mesalamine Rectal Suspension Enema and placebo were also statistically different in subgroups of patients on concurrent sulfasalazine and in those having an upper disease boundary between 5 and 20 or 20 and 40 cm. Significant differences between Mesalamine Rectal Suspension Enema and placebo were not achieved in those subgroups of patients on concurrent prednisone or with an upper disease boundary between 40 and 50 cm.
-
* Percent change for overall DAI only (calculated by taking the average of the change for each individual patient).
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Mesalamine Rectal Suspension Enema contains potassium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown but probably low. Sulfite sensitivity is seen more frequently in asthmatic or in atopic nonasthmatic persons.
Epinephrine is the preferred treatment for serious allergic or emergency situations even though epinephrine injection contains sodium or potassium metabisulfite with the above-mentioned potential liabilities. The alternatives to using epinephrine in a life-threatening situation may not be satisfactory. The presence of a sulfite(s) in epinephrine injection should not deter the administration of the drug for treatment of serious allergic or other emergency situations.
-
PRECAUTIONS
Mesalamine has been implicated in the production of an acute intolerance syndrome characterized by cramping, acute abdominal pain and bloody diarrhea, sometimes fever, headache and a rash; in such cases prompt withdrawal is required. The patient’s history of sulfasalazine intolerance, if any, should be re-evaluated. If a rechallenge is performed later in order to validate the hypersensitivity it should be carried out under close supervision and only if clearly needed, giving consideration to reduced dosage. In the literature one patient previously sensitive to sulfasalazine was rechallenged with 400 mg oral mesalamine; within eight hours she experienced headache, fever, intensive abdominal colic, profuse diarrhea and was readmitted as an emergency. She responded poorly to steroid therapy and two weeks later a pancolectomy was required.
Although renal abnormalities were not noted in the clinical trials with Mesalamine Rectal Suspension Enema, the possibility of increased absorption of mesalamine and concomitant renal tubular damage as noted in the preclinical studies must be kept in mind. Patients on Mesalamine Rectal Suspension Enema, especially those on concurrent oral products which liberate mesalamine and those with preexisting renal disease, should be carefully monitored with urinalysis, BUN (blood urea nitrogen), and creatinine studies.
In a clinical trial most patients who were hypersensitive to sulfasalazine were able to take mesalamine enemas without evidence of any allergic reaction. Nevertheless, caution should be exercised when mesalamine is initially used in patients known to be allergic to sulfasalazine. These patients should be instructed to discontinue therapy if signs of rash or fever become apparent.
While using Mesalamine Rectal Suspension Enema, some patients have developed pancolitis. However, extension of upper disease boundary and/or flare-ups occurred less often in the Mesalamine Rectal Suspension Enema treated group than in the placebo-treated group.
Worsening of colitis or symptoms of inflammatory bowel disease, including melena and hematochezia, may occur after commencing mesalamine.
Rare instances of pericarditis have been reported with mesalamine containing products including sulfasalazine. Cases of pericarditis have also been reported as manifestations of inflammatory bowel disease. In the cases reported with Mesalamine Rectal Suspension Enema, there have been positive rechallenges with mesalamine or mesalamine containing products. In one of these cases, however, a second rechallenge with sulfasalazine was negative throughout a 2-month follow-up. Chest pain or dyspnea in patients treated with Mesalamine Rectal Suspension Enema should be investigated with this information in mind. Discontinuation of Mesalamine Rectal Suspension Enema may be warranted in some cases, but rechallenge with mesalamine can be performed under careful clinical observation should the continued therapeutic need for mesalamine be present.
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Mesalamine caused no increase in the incidence of neoplastic lesions over controls in a 2-year study of Wistar rats fed up to 320 mg/kg/day of mesalamine admixed with diet. Mesalamine is not mutagenic to Salmonella typhimurium tester strains TA98, TA100, TA1535, TA1537, TA1538. There were no reverse mutations in an assay using E. coli strain WP2UVRA. There were no effects in an in vivo mouse micronucleus assay at 600 mg/kg and in an in vivo sister chromatid exchange at doses up to 610 mg/kg. No effects on fertility were observed in rats receiving up to 320 mg/kg/day. The oligospermia and infertility in men associated with sulfasalazine has very rarely been reported among patients treated with mesalamine.
Pregnancy
Teratologic studies have been performed in rats and rabbits at oral doses up to five and eight times respectively, the maximum recommended human dose, and have revealed no evidence of harm to the embryo or the fetus. There are, however, no adequate and well-controlled studies in pregnant women for either sulfasalazine or 5-ASA. Because animal reproduction studies are not always predictive of human response, 5-ASA should be used during pregnancy only if clearly needed.
-
ADVERSE REACTIONS
Clinical Adverse Experience
Mesalamine Rectal Suspension Enema is usually well tolerated. Most adverse effects have been mild and transient.
ADVERSE REACTIONS OCCURRING IN MORE THAN 0.1%OF MESALAMINE RECTAL SUSPENSION TREATED PATIENTS
(COMPARISON TO PLACEBO)SYMPTOM Mesalamine Rectal Suspension Enema
N = 815Placebo
N=128N % N % Abdominal Pain/Cramps/Discomfort
66
8.10
10
7.81
Headache
53
6.50
16
12.50
Gas/Flatulence
50
6.13
5
3.91
Nausea
47
5.77
12
9.38
Flu
43
5.28
1
0.78
Tired/Weak/Malaise/Fatigue
28
3.44
8
6.25
Fever
26
3.19
0
0.00
Rash/Spots
23
2.82
4
3.12
Cold/Sore Throat
19
2.33
9
7.03
Diarrhea
17
2.09
5
3.91
Leg/Joint Pain
17
2.09
1
0.78
Dizziness
15
1.84
3
2.34
Bloating
12
1.47
2
1.56
Back Pain
11
1.35
1
0.78
Pain on Insertion of Enema Tip
11
1.35
1
0.78
Hemorrhoids
11
1.35
0
0.00
Itching
10
1.23
1
0.78
Rectal Pain
10
1.23
0
0.00
Constipation
8
0.98
4
3.12
Hair Loss
7
0.86
0
0.00
Peripheral Edema
5
0.61
11
8.59
UTI/Urinary Burning
5
0.61
4
3.12
Rectal Pain/Soreness/Burning
5
0.61
3
2.34
Asthenia
1
0.12
4
3.12
Insomnia
1
0.12
3
2.34
In addition, the following adverse events have been identified during post-approval use of products which contain (or are metabolized to) mesalamine in clinical practice: nephrotoxicity, pancreatitis, fibrosing alveolitis, elevated liver enzymes, nephrogenic diabetes insipidus and intracranial hypertension. Cases of pancreatitis and fibrosing alveolitis have been reported as manifestations of inflammatory bowel disease as well. Published case reports and/or spontaneous post marketing surveillance have described rare instances of aplastic anemia, agranulocytosis, thrombocytopenia, eosinophilia, pancytopenia, neutropenia, oligospermia, and infertility in men. Anemia, leukocytosis, and thrombocytosis can be part of the clinical presentation of inflammatory bowel disease.
Hair Loss
Mild hair loss characterized by “more hair in the comb” but no withdrawal from clinical trials has been observed in 7 of 815 mesalamine patients but none of the placebo-treated patients. In the literature there are at least six additional patients with mild hair loss who received either mesalamine or sulfasalazine. Retreatment is not always associated with repeated hair loss.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
The usual dosage of Mesalamine Rectal Suspension Enema in 60 mL units is one rectal instillation (4 grams) once a day, preferably at bedtime, and retained for approximately eight hours. While the effect of Mesalamine Rectal Suspension Enema may be seen within 3 to 21 days, the usual course of therapy would be from 3 to 6 weeks depending on symptoms and sigmoidoscopic findings. Studies available to date have not assessed if Mesalamine Rectal Suspension Enema will modify relapse rates after the 6-week short-term treatment. Mesalamine Rectal Suspension Enema is for rectal use only.
Patients should be instructed to shake the bottle well to make sure the suspension is homogeneous. The patient should remove the protective sheath from the applicator tip. Holding the bottle at the neck will not cause any of the medication to be discharged. The position most often used is obtained by lying on the left side (to facilitate migration into the sigmoid colon); with the lower leg extended and the upper right leg flexed forward for balance. An alternative is the knee-chest position. The applicator tip should be gently inserted in the rectum pointing toward the umbilicus. A steady squeezing of the bottle will discharge most of the preparation. The preparation should be taken at bedtime with the objective of retaining it all night. Patient instructions are included with every seven units.
-
HOW SUPPLIED
Mesalamine Rectal Suspension Enema for rectal administration is an off-white to tan colored suspension. Each disposable enema bottle contains 4 grams of mesalamine in 60 mL aqueous suspension. Enema bottles are supplied in boxed, foil-wrapped trays as follows:
- Carton of 7 Bottles: NDC: 62559-420-07
Mesalamine Rectal Suspension Enemas are for rectal use only.
KEEP OUT OF REACH OF CHILDREN
Patient instructions are included.
Storage
Store at controlled room temperature 20° to 25°C (68° to 77°F); excursions permitted, please refer to current USP. Once the foil-wrapped unit of seven bottles is opened, all enemas should be used promptly as directed by your physician. Contents of enemas removed from the foil pouch may darken with time. Slight darkening will not affect potency, however, enemas with dark brown contents should be discarded.
NOTE: Mesalamine Rectal Suspension Enema will cause staining of direct contact surfaces, including but not limited to fabrics, flooring, painted surfaces, marble, granite, vinyl, and enamel. Take care in choosing a suitable location for administration of this product.
Rx only
Distributed by:
ANI Pharmaceuticals, Inc.
Baudette, MN 56623

9819 Rev 07/17
For Medical Inquiries, Call Toll Free: 1-800-308-6755
-
PATIENT INSTRUCTIONS
How to Use this Medication.
Best results are achieved if the bowel is emptied immediately before the medication is given.
NOTE: Mesalamine Rectal Suspension Enema will cause staining of direct contact surfaces, including but not limited to fabrics, flooring, painted surfaces, marble, granite, vinyl, and enamel. Take care in choosing a suitable location for administration of this product.
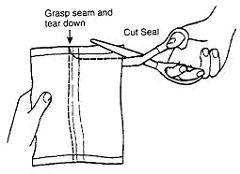
1. Remove the Bottles
- 1. Remove the bottles from the protective foil pouch by tearing or by using scissors as shown, being careful not to squeeze or puncture bottles. Mesalamine Rectal Suspension Enema is an off-white to tan colored suspension. Once the foil-wrapped unit of seven bottles is opened, all enemas should be used promptly as directed by your physician. Contents of enemas removed from the foil pouch may darken with time. Slight darkening will not affect potency, however, enemas with dark brown contents should be discarded.
2. Prepare the Medication for Administration
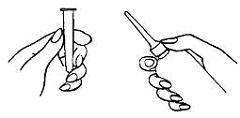
- 1. Shake the bottle well to make sure that the medication is thoroughly mixed.
- 2. Remove the protective sheath from the applicator tip. Hold the bottle at the neck so as not to cause any of the medication to be discharged.
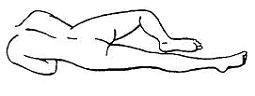
3. Assume the Correct Body Position
- 1. Best results are obtained by lying on the left side with the left leg extended and the right leg flexed forward for balance.
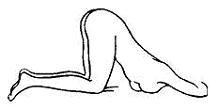
- 1. An alternative to lying on the left side is the “knee-chest” position as shown here.
4. Administer the Medication
- 1. Gently insert the lubricated applicator tip into the rectum to prevent damage to the rectal wall, pointed slightly toward the navel.
- 2. Grasp the bottle firmly, then tilt slightly so that the nozzle is aimed toward the back, squeeze slowly to instill the medication. Steady hand pressure will discharge most of the medication. After administering, withdraw and discard the bottle.
- 1. Remain in position for at least 30 minutes to allow thorough distribution of the medication internally. Retain the medication all night, if possible.
Rx only
Distributed by:
ANI Pharmaceuticals, Inc.
Baudette, MN 56623

9819 Rev 07/17
For Medical Inquiries, Call Toll Free: 1-800-308-6755
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Mesalamine Rectal Suspension Enema, 4 g/60 mL
NDC: 62559-420-07
FOR RECTAL USE ONLY
Rx only
7 x 60 mL Unit-Dose Bottles

-
INGREDIENTS AND APPEARANCE
MESALAMINE
mesalamine enemaProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62559-420 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MESALAMINE (UNII: 4Q81I59GXC) (MESALAMINE - UNII:4Q81I59GXC) MESALAMINE 4 g in 60 mL Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL OR ALLYL SUCROSE CROSSLINKED) (UNII: K6MOM3T5YL) EDETATE DISODIUM (UNII: 7FLD91C86K) POTASSIUM ACETATE (UNII: M911911U02) POTASSIUM METABISULFITE (UNII: 65OE787Q7W) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM BENZOATE (UNII: OJ245FE5EU) PETROLATUM (UNII: 4T6H12BN9U) Product Characteristics Color WHITE (off-white to tan) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62559-420-07 7 in 1 BOX 05/13/2016 1 NDC: 62559-420-11 60 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA019618 05/13/2016 Labeler - ANI Pharmaceuticals, Inc. (145588013)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.