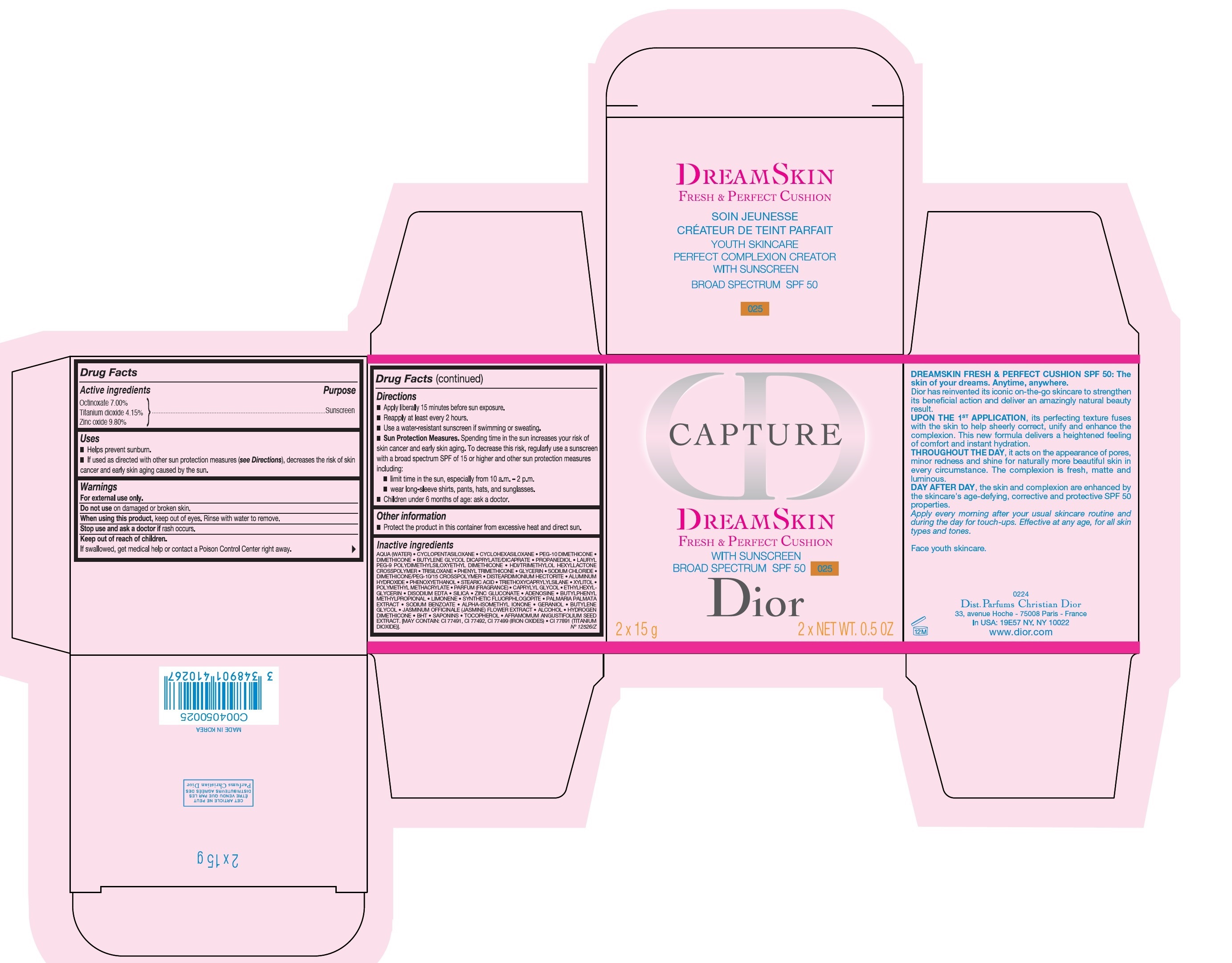
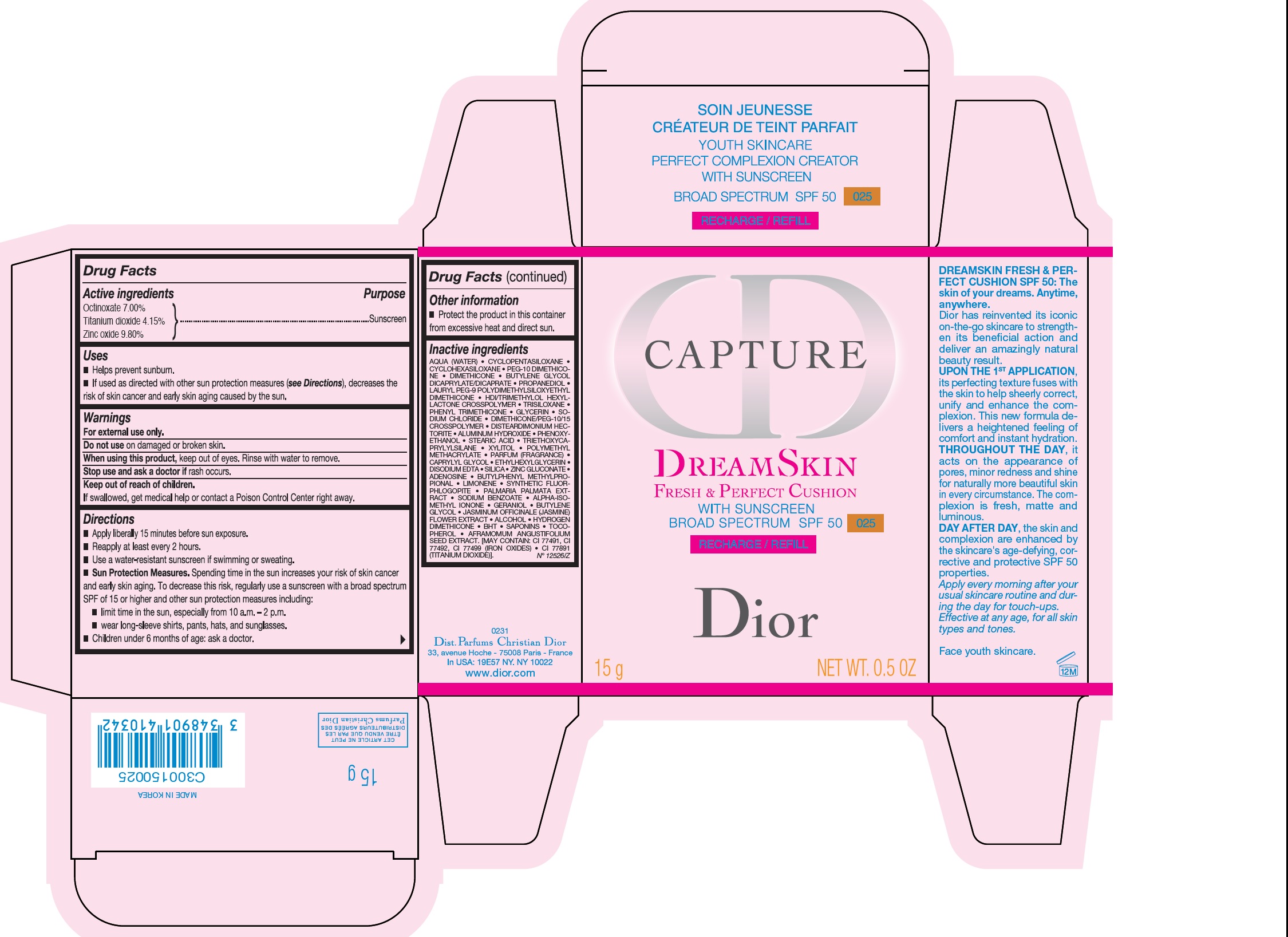
CAPTURE DREAMSKIN FRESH AND PERFECT CUSHION WITH SUNSCREEN BROAD SPECTRUM SPF 50 025- octinoxate, titanium dioxide, zinc oxide emulsion
CAPTURE DREAMSKIN FRESH AND PERFECT CUSHION WITH SUNSCREEN BROAD SPECTRUM SPF 50 025 by
Drug Labeling and Warnings
CAPTURE DREAMSKIN FRESH AND PERFECT CUSHION WITH SUNSCREEN BROAD SPECTRUM SPF 50 025 by is a Otc medication manufactured, distributed, or labeled by Parfums Christian Dior. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients
- Uses
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decreases this risk, regularly use a sunsreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses.
- Children under 6 months of age: ask a doctor.
- Other information
-
Inactive ingredients
AQUA (WATER), CYCLOPENTASILOXANE, CYCLOHEXASILOXANE, PEG-10 DIMETHICONE, DIMETHICONE, BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE, PROPANEDIOL, LAURYL PEG-9 POLYDIMETHYLSILOXETHYL DIMETHICONE, HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER, TRISILOXANE, PHENYL TRIMETHICONE, GLYCERIN, SODIUM CHLORIDE, DIMETHICONE/PEG-/ CROSSPOLYMER, DISTEARDIMONIUM HECTORITE, ALUMINUM HYDROXIDE, PHENOXYETHANOL, STEARIC ACID, XYLITOL, POLYMETHYL METHACRYLATE, PARFUM (FRAGRANCE), CAPRYLYL GLYCOL, ETHYLHEXYLGLYCERIN, DISODIUM EDTA, SILICA, ZINC GLUCONATE, ADENOSINE, BUTYLPHENYL METHYLPROPIONAL, LIMONENE, SYNTHETIC FLUORPHLOGOPITE, PALMARIA PALMATA EXTRACT, SODIUM BENZOATE, ALPHA-ISOMETHYL IONONE, GERANIOL, BUTYLENE GLYCOL, JASMINUM OFFICINALE (JASMINE) FLOWER EXTRACT, ALCOHOL, HYDROGEN DIMETHICONE, BHT, SAPONINS, TOCOPHEROL, AFRAMOMUM ANGUSTIFOLIUM SEED EXTRACT. [MAY CONTAIN: CI 77491, CI 77492, CI 77499 (IRON OXIDES), CI 77891 (TITANIUM DIOXIDE)].
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
CAPTURE DREAMSKIN FRESH AND PERFECT CUSHION WITH SUNSCREEN BROAD SPECTRUM SPF 50 025
octinoxate, titanium dioxide, zinc oxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 61957-2903 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 70 mg in 1 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 41.5 mg in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 98 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) PROPANEDIOL (UNII: 5965N8W85T) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) TRISILOXANE (UNII: 9G1ZW13R0G) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) GLYCERIN (UNII: PDC6A3C0OX) SODIUM CHLORIDE (UNII: 451W47IQ8X) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) XYLITOL (UNII: VCQ006KQ1E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ZINC GLUCONATE (UNII: U6WSN5SQ1Z) ADENOSINE (UNII: K72T3FS567) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) LIMONENE, (+)- (UNII: GFD7C86Q1W) DULSE (UNII: 7832HOY4ZQ) SODIUM BENZOATE (UNII: OJ245FE5EU) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) GERANIOL (UNII: L837108USY) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) JASMINUM OFFICINALE FLOWER (UNII: 0Q8K841432) ALCOHOL (UNII: 3K9958V90M) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) TOCOPHEROL (UNII: R0ZB2556P8) AFRAMOMUM ANGUSTIFOLIUM SEED (UNII: OSF83Q896J) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61957-2903-0 2 in 1 CARTON 07/01/2018 1 15 g in 1 JAR; Type 0: Not a Combination Product 2 NDC: 61957-2903-1 1 in 1 CARTON 07/01/2018 2 15 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 07/01/2018 Labeler - Parfums Christian Dior (275252245)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.