ANTI DIARRHEAL- loperamide hcl tablet
Anti Diarrheal by
Drug Labeling and Warnings
Anti Diarrheal by is a Otc medication manufactured, distributed, or labeled by CHAIN DRUG MARKETING ASSOCIATION INC, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
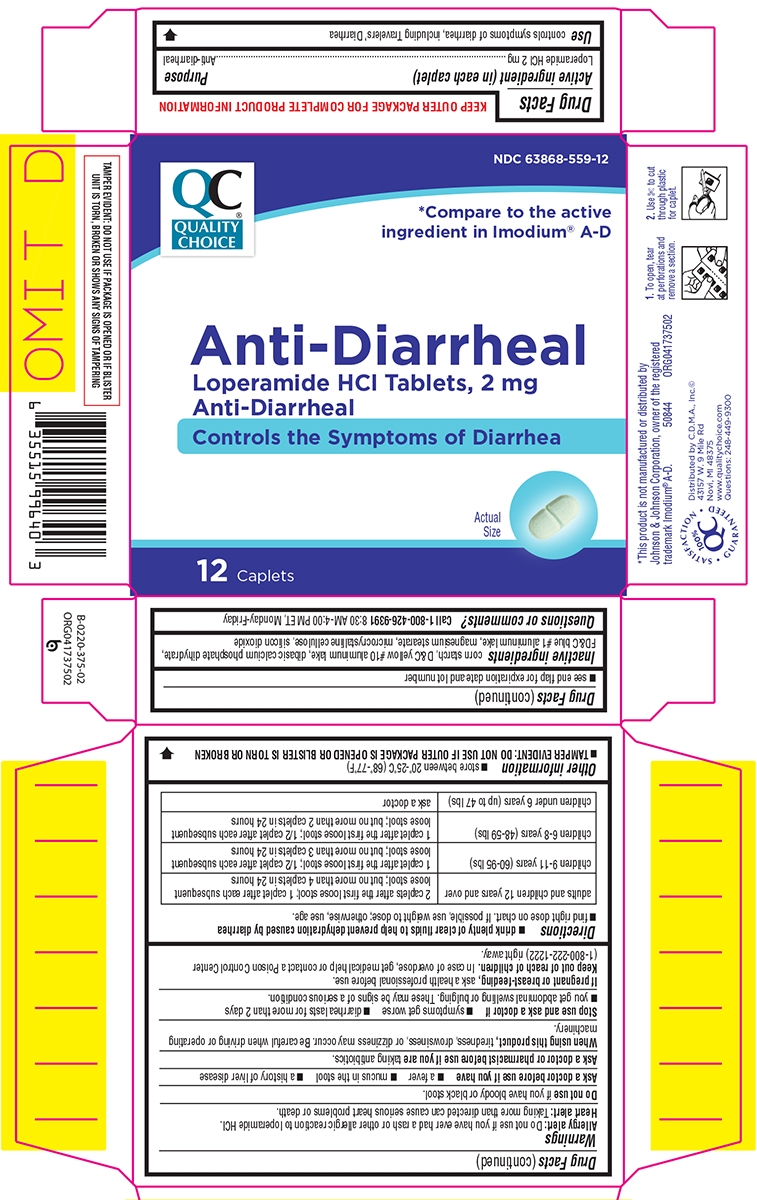
- Active ingredient (in each caplet)
- Purpose
- Use
-
Warnings
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCl.
Heart alert: Taking more than directed can cause serious heart problems or death.
Ask a doctor before use if you have
- a fever
- mucus in the stool
- a history of liver disease
- a history of abnormal heart rhythm
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Loperamide may interact with certain prescription drugs.
When using this product
tiredness, drowsiness, or dizziness may occur. Be careful when driving or operating machinery.
- a fever
-
Directions
-
drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
adults and children 12 years and over 2 caplets after the first loose stool; 1 caplet after each subsequent
loose stool; but no more than 4 caplets in 24 hours
children 9-11 years (60-95 lbs)
1 caplet after the first loose stool; 1/2 caplet after each subsequent
loose stool; but no more than 3 caplets in 24 hours
children 6-8 years (48-59 lbs)
1 caplet after the first loose stool; 1/2 caplet after each subsequent
loose stool; but no more than 2 caplets in 24 hours
children 2-5 years (34-47 lbs) ask a doctor children under 2 years (up to 33 lbs)
do not use
-
drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
QC®
Quality
ChoiceNDC: 63868-559-24
*Compare to the active ingredient in IMODIUM® A-D
Anti-Diarrheal
Loperamide HCl Tablets, 2 mg
Anti-DiarrhealControls the Symptoms of Diarrhea
Actual Size
24 Caplets
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed by Johnson & Johnson Corporation, owner of the registered trademark IMODIUM® A-D.
Distributed by C.D.M.A., Inc.©
43157 W. 9 Mile Rd
Novi, MI 48375
www.qualitychoice.com
Questions: 800-935-236250844 REV0619A37508
Quality Choice 44-375
-
INGREDIENTS AND APPEARANCE
ANTI DIARRHEAL
loperamide hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 63868-559 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LOPERAMIDE HYDROCHLORIDE (UNII: 77TI35393C) (LOPERAMIDE - UNII:6X9OC3H4II) LOPERAMIDE HYDROCHLORIDE 2 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) Product Characteristics Color GREEN (Light) Score 2 pieces Shape OVAL Size 10mm Flavor Imprint Code 44;375 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63868-559-24 4 in 1 CARTON 05/03/2005 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 63868-559-12 2 in 1 CARTON 05/03/2005 2 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC: 63868-559-60 1 in 1 CARTON 05/03/2005 3 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076497 05/03/2005 Labeler - CHAIN DRUG MARKETING ASSOCIATION INC (011920774) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 MANUFACTURE(63868-559) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(63868-559) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 PACK(63868-559) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(63868-559) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(63868-559)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.