JELMYTO by UroGen Pharma, Inc. / UroGen Pharma, Ltd. JELMYTO- mitomycin kit
JELMYTO by
Drug Labeling and Warnings
JELMYTO by is a Prescription medication manufactured, distributed, or labeled by UroGen Pharma, Inc., UroGen Pharma, Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use JELMYTO safely and effectively. See full prescribing information for JELMYTO.
JELMYTO™ (mitomycin) for pyelocalyceal solution
Initial U.S. Approval: 1974INDICATIONS AND USAGE
JELMYTO is an alkylating drug indicated for the treatment of adult patients with low-grade Upper Tract Urothelial Cancer (LG-UTUC). (1)
DOSAGE AND ADMINISTRATION
- JELMYTO is for pyelocalyceal use only and not for intravenous use, topical use, or oral administration. (2.1)
- Administer 1.3 g of sodium bicarbonate orally the evening prior to, the morning of, and 30 minutes prior to instillation procedure (total of 3.9 g). (2.1)
- The dose of JELMYTO to be instilled is 4 mg per mL via ureteral catheter or nephrostomy tube, with total instillation volume based on volumetric measurements using pyelography, not to exceed 15 mL (60 mg of mitomycin). (2.2)
- Instill JELMYTO once weekly for six weeks. For patients with a complete response 3 months after JELMYTO initiation, JELMYTO instillations may be administered once a month for a maximum of 11 additional instillations. (2.2)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- Perforation of the bladder or upper urinary tract. (4)
WARNINGS AND PRECAUTIONS
- Ureteric Obstruction: Ureteric obstruction may occur. Monitor patients for signs and symptoms of ureteric obstruction. Transient or long-term ureteral stents or alternative procedures may be required. Withhold or permanently discontinue JELMYTO based on the severity of the ureteric obstruction. (5.1)
- Bone Marrow Suppression: Thrombocytopenia and neutropenia may occur. Monitor blood counts. Withhold or permanently discontinue JELMYTO based on the severity. (5.2)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise of potential risk to a fetus and to use effective contraception. (5.3, 8.1, 8.3)
ADVERSE REACTIONS
The most common adverse reactions (≥ 20%) are ureteric obstruction, flank pain, urinary tract infection, hematuria, renal dysfunction, fatigue, nausea, abdominal pain, dysuria, and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact UroGen Pharma at 1-855-987-6436 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Recommended Dosage
2.3 Preparation and Handling
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Ureteric Obstruction
5.2 Bone Marrow Suppression
5.3 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
See the Instructions for Administration provided separately.
JELMYTO is for pyelocalyceal use only. JELMYTO is not for intravenous use, topical use, or oral administration. Prior to every instillation, instruct the patient to take 1.3 g of sodium bicarbonate orally the evening prior to, the morning of, and 30 minutes prior to the instillation procedure (total of 3.9 g).
General anesthesia, local anesthesia, sedation, prophylactic antibiotics and/or antihistamines may be used at the discretion of the treating urologist. If the patient is to be anesthetized, advise the patient not to take sodium bicarbonate within 30 minutes prior to the treatment.
Consider withholding diuretics one day prior to instillation until 4 hours post-instillation.
When instilling JELMYTO, the entire syringe must be emptied within one minute.
Advise patients that JELMYTO may discolor urine to a violet to blue color following the instillation procedure. Advise patients to avoid contact with urine for at least six hours post-instillation, to void urine sitting on a toilet, and to flush the toilet several times after use.
2.2 Recommended Dosage
The dose of JELMYTO to be instilled is 4 mg per mL via ureteral catheter or a nephrostomy tube, with total instillation volume based on volumetric measurements using pyelography, not to exceed 15 mL (60 mg of mitomycin).
Instill JELMYTO once weekly for six weeks. For patients with a complete response 3 months after JELMYTO initiation, JELMYTO instillations may be administered once a month for a maximum of 11 additional instillations.
2.3 Preparation and Handling
See the Instructions for Pharmacy for preparation provided separately.
JELMYTO is a cytotoxic drug. Follow applicable special handling and disposal procedures.1
JELMYTO must be prepared under chilled conditions. Once reconstituted, the admixture will have a concentration of 4 mg of mitomycin per mL and will appear as a viscous liquid for instillation. Reconstituted JELMYTO has reverse thermal properties with a gelation point of approximately 19°C (66°F). Reconstituted JELMYTO should be instilled as soon as possible after reconstitution. If immediate instillation is not possible store reconstituted JELMYTO at 20°C to 25°C (68°F to 77°F) for up to 8 hours. JELMYTO will appear as a semisolid gel when stored under these conditions. Protect reconstituted JELMYTO from light.
JELMYTO must be instilled as a chilled solution using a Uroject12 Lever, a Luer lock syringe, and a ureteral catheter with molded Luer lock connector. Once chilled at -3°C to 5°C (27°F to 41°F), JELMYTO will convert to a viscous liquid for instillation and is stable for up to 1 additional hour. Reconstituted JELMYTO must be instilled within 1 hour after it is converted to a viscous liquid.
-
3 DOSAGE FORMS AND STRENGTHS
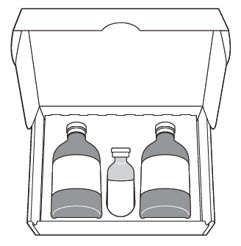
For pyelocalyceal solution: A single-dose carton containing the following:
- Two 40 mg (each) single-dose vials of sterile, lyophilized, grey to greyish-purple, cake or powder of mitomycin for pyelocalyceal solution
- One single-dose vial of 20 mL of sterile, clear, colorless, gel with or without bubbles at room temperature or clear, colorless liquid at 2°C to 8°C (36°F to 46°F), to be used as a vehicle for reconstitution
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Ureteric Obstruction
Ureteric obstruction, including ureteral stenosis and hydronephrosis, occurred in patients receiving JELMYTO.
In the OLYMPUS study, ureteric obstruction was reported in 58% (n=41) of patients receiving JELMYTO, including 17% (n=12) of patients who experienced Grade 3 obstruction. The median time to first onset was 72 days (range: 15-462). Interventions in the 41 patients experiencing ureteric obstruction included ureteral stent placement (88%), balloon dilatation (32%), and nephroureterectomy (4.9%). In the 36 patients who required ureteral stent placement, the median duration of indwelling stents was 51 days (range: 1-292). Ureteric obstruction did not resolve or resolved with sequelae in 51% (n=21) of these patients. Of the 41 patients who experienced ureteric obstruction, 17% (n=7) experienced Grades 1-2 increase in serum creatinine.
In the 42 patients who only received JELMYTO during the treatment phase (no maintenance therapy), ureteric obstruction was reported in 40% (n=17).
Monitor patients for signs and symptoms of ureteric obstruction, including flank pain, and fever, and for changes in renal function. Patients who experience obstruction may require transient or long-term ureteral stents or alternative procedures. Withhold or permanently discontinue JELMYTO based on the severity of ureteric obstruction.
5.2 Bone Marrow Suppression
The use of JELMYTO can result in bone marrow suppression, particularly thrombocytopenia and neutropenia. In the OLYMPUS study, Grade 3 thrombocytopenia occurred in two patients and Grade 3 neutropenia in one patient. Gross extravasation of JELMYTO via urinary tract perforation or impaired mucosa was not observed in these patients. The following tests should be obtained prior to each treatment: Platelet count, white blood cell count differential and hemoglobin. Withhold JELMYTO for Grade 2 thrombocytopenia or neutropenia. Permanently discontinue for Grade 3 or greater thrombocytopenia or neutropenia.
5.3 Embryo-Fetal Toxicity
Based on findings in animals and mechanism of action, JELMYTO can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of mitomycin resulted in teratogenicity. Advise females of reproductive potential to use effective contraception during treatment with JELMYTO and for 6 months following the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with JELMYTO and for 3 months following the last dose [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.1)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Ureteric Obstruction [see Warnings and Precautions (5.1)]
- Bone Marrow Suppression [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect rates observed in practice.
The safety of JELMYTO was evaluated in OLYMPUS, an open-label, single-arm study in 71 patients with LG-UTUC [see Clinical Studies (14)]. For the 71 patients treated with JELMYTO during the treatment period, the median number of instillations was 6 (range: 3-6). Following initial treatment, 29 patients were treated with up to 11 doses of maintenance instillations, with a median of 6 instillations (range: 0-11).
Serious adverse reactions occurred in 37% of patients who received JELMYTO. Serious adverse reactions in > 3% of patients included ureteric obstruction (including ureteric stenosis and hydronephrosis), flank pain, and urosepsis. Two deaths occurred due to cerebrovascular accident and failure to thrive.
JELMYTO was permanently discontinued due to an adverse reaction in 16 (23%) patients, including 11 patients who discontinued during the treatment phase and 5 who discontinued during the maintenance phase. Adverse reactions resulting in study drug discontinuation of JELMYTO in > 3% of patients who received JELMYTO included ureteric obstruction.
Dosage interruptions due to an adverse reaction occurred in 34% of patients who received JELMYTO. Adverse reactions requiring dosage interruption in > 3% of patients who received JELMYTO included renal dysfunction, ureteric obstruction, urinary tract infection, and flank pain.
The most common adverse reactions (≥ 20%) reported were ureteric obstruction, flank pain, urinary tract infection, hematuria, renal dysfunction, fatigue, nausea, abdominal pain, dysuria, and vomiting.
Table 1 summarizes the adverse reactions in OLYMPUS.
Table 1: Adverse Reactions (≥ 10% All Grades) in Patients Who Received JELMYTO in OLYMPUS
Adverse ReactionJELMYTO*
(n=71)All Grades
(%)Grade ≥ 3
(%)- * Graded per National Cancer Institute Common Terminology Criteria for Adverse Events. Version 5.0 (NCI CTCAE v5)
- † Includes hydronephrosis, obstructive uropathy, pelvi-ureteric obstruction, ureteric obstruction, ureteric stenosis, and urinary tract obstruction.
- ‡ Includes flank pain and back pain.
- § Includes urinary tract infection, pyelonephritis, and urinary tract infection fungal.
- ¶ Includes hematuria and hemorrhage urinary tract.
- # Includes renal impairment, acute kidney injury, and renal failure.
- Þ Includes abdominal pain and abdominal pain lower.
- ß Includes asthenia and fatigue.
Renal and urinary disorders Ureteric Obstruction† 58 17 Ureteric stenosis 44 8 Hydronephrosis 18 6 Pelvi-ureteric obstruction 6 1.4 Urinary tract obstruction 6 1.4 Ureteric obstruction 2.8 1.4 Obstructive uropathy 1.4 0 Flank pain‡ 39 2.8 Urinary tract infection § 34 4.2 Hematuria¶ 32 2.8 Renal dysfunction# 25 2.8 Dysuria 21 0 Pollakiuria 13 0 Gastrointestinal disorders Nausea 24 1.4 Abdominal painÞ 23 1.4 Vomiting 20 4.2 General disorders and administration site conditions Fatigueß 24 1.4 Chills 11 0 Pyrexia 11 0 Blood and lymphatic system disorders Anemia 13 0 Skin and subcutaneous tissue disorders Pruritus 13 0 Selected clinically relevant adverse reactions in < 10% and ≥ 2% of patients who received JELMYTO in OLYMPUS include urinary tract inflammation, bladder spasm, urosepsis, hypersensitivity, and instillation site pain.
Table 2 summarizes the laboratory abnormalities in OLYMPUS.
Table 2: Select Laboratory Abnormalities (> 10%) Worsening from Baseline in Patients Who Received JELMYTO in OLYMPUS Laboratory Abnormality* JELMYTO All Grades (%) Grades ≥ 3 (%) - * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available.
Hematology Anemia 37 0 Lymphopenia 21 2.9 Thrombocytopenia 21 2.8 Chemistry Estimated Glomerular Filtration Rate 37 10 Creatinine Increased 32 0 Hypoalbuminemia 30 2.8 Hypocalcemia 17 0 Hyperuricemia 16 16 Hyperkalemia 13 1.4 -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings in animals and mechanism of action, JELMYTO can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on JELMYTO use in pregnant women to inform the drug-associated risk. In animal reproduction studies, administration of mitomycin resulted in teratogenicity (see Data). Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% - 4% and 15% - 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of mitomycin in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with JELMYTO and for 1 week following the last dose.
8.3 Females and Males of Reproductive Potential
JELMYTO can cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating JELMYTO.
8.5 Geriatric Use
Of the total number of patients in the OLYMPUS trial, 75% (53 patients) were 65 years of age and over and 37% (26 patients) were 75 year of age and over. Clinical studies of JELMYTO did not include sufficient numbers of younger patients less than 65 years old to determine whether they respond differently from older patients.
-
11 DESCRIPTION
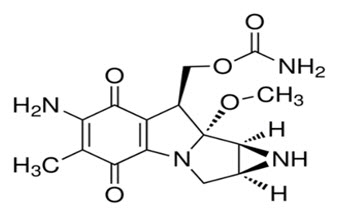
Mitomycin (also known as mitomycin-C) is an alkylating drug isolated from the broth of Streptomyces caespitosus. Mitomycin is a blue-violet crystalline powder with a molecular formula of C15H18N4O5, and a molecular weight of 334.33. Its chemical name is 7-amino-9α-methoxymitosane and it has the following structural formula:
Mitomycin is heat stable, has a high melting point, and is freely soluble in organic solvents.
JELMYTO is supplied in a single-dose carton containing two vials of sterile lyophilized mitomycin for pyelocalyceal solution, 40 mg each, and one vial of 20 mL of sterile hydrogel, to be used as a vehicle for reconstitution.
Mitomycin for pyelocalyceal solution is a sterile, lyophilized, grey to greyish-purple, cake or powder that contains mitomycin 40 mg and mannitol 80 mg in each vial.
Sterile hydrogel is a sterile, clear, colorless, gel with or without bubbles at room temperature or clear, colorless liquid at 2°C to 8°C (36°F to 46°F), which contains 0.04 g hydroxypropyl methylcellulose, 5.67 g poloxamer, 0.21 g polyethylene glycol, and water for injection in each vial.
Once reconstituted, JELMYTO is a clear, purple, viscous liquid at 2°C to 8°C (36°F to 46°F) or semisolid gel at room temperature with a concentration of 4 mg per mL of mitomycin, which may contain a few visible particles and have a pH between 6.0 and 8.0.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mitomycin inhibits the synthesis of deoxyribonucleic acid (DNA). The guanine and cytosine content correlates with the degree of mitomycin-induced cross-linking. At high concentrations of the drug, cellular RNA and protein synthesis are also suppressed.
12.2 Pharmacodynamics
There is insufficient data to characterize an exposure-response relationship or time course of pharmacodynamic response for mitomycin.
12.3 Pharmacokinetics
Absorption
The systemic exposure of mitomycin following instillation of up to 60 mg of mitomycin as JELMYTO into the pyelocalyceal system was evaluated pre-instillation and hourly for up to six hours post- instillation in six patients. The concentrations of mitomycin in plasma were variable and ranged from 2.43 to 12.80 ng/mL over the course of treatment; the mean Cmax was 6.24 ng/mL, which is estimated to be less than 1% of the expected Cmax after intravenous administration.
Elimination
Following instillation into the pyelocalyceal system, JELMYTO forms a semisolid gel which dissolves from normal kidney urine flow releasing mitomycin for up to 4 to 6 hours. Mitomycin is eliminated unchanged in the urine. Systemically absorbed mitomycin is rapidly cleared from the serum and approximately 10% is excreted unchanged in the urine.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Adequate long-term studies in animals to evaluate carcinogenic potential from instillation of mitomycin into the pyelocalyceal system have not been conducted. Mitomycin has been found to be carcinogenic in rats and mice. At doses approximating the recommended intravenous clinical dose in humans, mitomycin produced a greater than 100% increase in tumor incidence in male Sprague-Dawley rats, and a greater than 50% increase in tumor incidence in female Swiss mice.
The effect of JELMYTO on fertility is unknown.
-
14 CLINICAL STUDIES
The efficacy of JELMYTO is based on the results of the ongoing study OLYMPUS (NCT02793128), an open-label, single-arm, multicenter trial that enrolled 71 patients with treatment-naïve or recurrent non-invasive low-grade upper tract urothelial cancer (LG-UTUC) with at least one measurable papillary tumor 5 to ≤ 15 mm located above the ureteropelvic junction; patients who had larger tumors could have had tumor debulking prior to treatment, in order to meet the criteria. Patients were excluded from the trial for a history of carcinoma in situ (CIS) in the urinary tract, invasive urothelial carcinoma within 5 years, high grade papillary urothelial carcinoma within 2 years; or for BCG treatment within 6 months of JELMYTO treatment. Following biopsy and prior to treatment, patients were required to have at least one remaining visible tumor with a diameter of at least 5 mm.
Patients received JELMYTO 4 mg per mL via ureteral catheter or nephrostomy tube with total instillation volume based on individualized volumetric measurements using pyelography with the intent to fill the renal pelvis. Patients were treated with 6 instillations once a week. Patients who maintained a complete response (CR) after the initial treatment period were allowed to proceed to the follow-up period. During the initial treatment period, 71 patients were treated with JELMYTO, of whom 41 were subsequently continued in the follow-up period. During the follow-up period, 29 patients received at least one dose of maintenance therapy.
The baseline demographic and disease characteristics for the trial population were: median age 71 years (range: 42-87 years); 68% male; 87% White; 90% Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0 or 1 and 10% ECOG PS 2. The median number of papillary lesions subsequent to debulking and/or biopsy and prior to treatment was 1 lesion (range: 1, 5), the median diameter of the largest lesion was 8.0 mm (range: 5.0, 15.0), and the median total visible tumor burden was 10.0 mm (range: 5.0, 25.0). Twenty-six (37%) patients underwent tumor debulking during the six weeks preceding enrollment. Of 71 enrolled patients, 48% had tumors located in regions not amenable to endoscopic resection. General anesthesia was used in 37% of patients for at least one instillation during the treatment period and for 61% of patients for at least one instillation during the follow-up period.
The major efficacy outcome measures were CR and durability of CR at 12 months after determination of CR based on ureteroscopic and local pathology assessment. CR was defined as complete absence of tumor lesions at 3 months after initiation of JELMYTO by urine cytology and ureteroscopy. Biopsy was performed if warranted. Durability of response in patients with a CR was evaluated at 3, 6, 9 and 12 months following the initial assessment. Assessment of durability of CR subsequent to these evaluations was performed per local standards of care.
Forty-one patients achieved CR in the study. At the 12-month time point for assessment of durability, 19 remained in CR, 7 had experienced recurrence of disease, and 9 patients continued to be followed for the 12-month duration of response. Efficacy results are provided in Table 3.
Table 3: Efficacy Results for OLYMPUS Efficacy Parameter JELMYTO
N=71NR = not reached
+ denotes ongoing response.- * CR = Complete absence of tumor lesions at 3 months after initiation of treatment.
- † 12 months ± 2 weeks from CR evaluation.
Complete Response (CR)*, n (%)
(95% CI)41 (58%)
(45%, 69%)Duration of Response N=41 Median duration of response in months (range) NR (0, 18.8+) Patients remaining in CR at 12-month visit† 19 (46%) - 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
JELMYTO single-dose carton – NDC: 72493-103-03
A carton containing the following:
- Two 40 mg (each) single-dose vials of mitomycin for pyelocalyceal solution supplied as a sterile, lyophilized, grey to greyish-purple, cake or powder. (NDC: 72493-101-40)
- One 20 mL single-dose vial of sterile hydrogel supplied as a sterile, clear, colorless, gel with or without bubbles at room temperature or clear, colorless liquid at 2°C to 8°C (36°F to 46°F), to be used as a vehicle for reconstitution. (NDC: 72493-102-20)
16.2 Storage and Handling
Store the JELMYTO carton at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature]. Avoid excessive heat over 40°C (104°F).
JELMYTO is a cytotoxic drug. Follow applicable special handling and disposal procedures.1
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Ureteric Obstruction
Inform patients that ureteric obstruction may occur, and ureteral stents or alternative procedures may be required during treatment with JELMYTO. Advise patients to contact their healthcare provider immediately if signs and symptoms of ureteric obstruction, including flank pain and/or fever, occur [see Warnings and Precautions (5.1)].
Bone Marrow Suppression
Inform patients that JELMYTO may decrease blood counts such as white blood cells and platelets. Thus, it is important that periodic assessment of their blood count be performed to detect the development of neutropenia and thrombocytopenia [see Warnings and Precautions (5.2)].
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare providers of a known or suspected pregnancy [see Warnings and Precautions (5.3) and Use in Specific Population (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with JELMYTO and for 6 months following the last dose [see Use in Specific Populations (8.3)].
Advise male patients with female partners of reproductive potential to use effective contraception during treatment with JELMYTO and for 3 months following the last dose [see Use in Specific Populations (8.3)].
Lactation
Advise women not to breastfeed during treatment with JELMYTO and for 1 week following the last dose [see Use in Specific Populations (8.2)].
Important Post-Treatment Instructions [see Dosage and Administration (2.1)].
Advise patients that JELMYTO contains mitomycin which is a violet to blue color and may discolor urine following the instillation procedure.
Advise patients to avoid contact with urine for at least six hours post-instillation.
Advise patients to void sitting on a toilet, flush the toilet several times after use, and to wash hands, perineum or glans with soap and water after each instillation procedure.
Advise patients to wash clothing soiled with urine promptly and separately from other clothing.
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: April 2020 Patient Information
JELMYTO™ (jel-MYE-toe)
(mitomycin)
for pyelocalyceal solutionWhat is JELMYTO?
JELMYTO is a prescription medicine used to treat adults with a type of cancer of the lining of the upper urinary tract including the kidney called low-grade Upper Tract Urothelial Cancer (LG-UTUC).
It is not known if JELMYTO is safe and effective for use in children.Who should not receive JELMYTO?
Do not receive JELMYTO if you have a hole or tear (perforation) of your bladder or upper urinary tract.Before receiving JELMYTO, tell your healthcare provider about all your medical conditions, including if you: - are pregnant or plan to become pregnant. JELMYTO can harm your unborn baby. You should not become pregnant during treatment with JELMYTO. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with JELMYTO.
Females who are able to become pregnant:- Your healthcare provider will check to see if you are pregnant before starting treatment with JELMYTO.
- You should use effective birth control (contraception) during treatment with JELMYTO and for 6 months after the last dose.
- Talk to your healthcare provider if you have questions about birth control options that are right for you.
- If you have a female partner who is able to become pregnant, you should use effective birth control (contraception) during treatment with JELMYTO and for 3 months after the last dose.
- are breastfeeding or plan to breastfeed. It is not known if JELMYTO passes into your breast milk. Do not breastfeed during treatment with JELMYTO and for 1 week after the last dose.
Especially tell your healthcare provider if you take water pills (diuretic).How will I receive JELMYTO? - Your healthcare provider will tell you to take a medicine called sodium bicarbonate before each JELMYTO treatment. Your healthcare provider will provide instructions about how and when to take this.
- JELMYTO will be given to you by your healthcare provider.
- You will receive JELMYTO 1 time a week for 6 weeks. It is important that you receive all 6 doses of JELMYTO according to your healthcare provider's instructions. If you miss any appointments, call your healthcare provider as soon as possible to reschedule your appointment. Your healthcare provider may recommend up to an additional 11 monthly doses.
- JELMYTO is given to your kidney through a tube called a catheter.
- During treatment with JELMYTO, your healthcare provider may tell you to take additional medicines or change how you take your current medicines. Ask your healthcare provider if you have any questions.
- JELMYTO may cause your urine color to change to a violet to blue color.
- Avoid contact between your skin and urine for at least 6 hours.
- To urinate, males and females should sit on a toilet and flush the toilet several times after you use it.
- After going to the bathroom, wash your hands, your inner thighs, and genital area well with soap and water.
- Clothing that comes in contact with urine should be washed right away and washed separately from other clothing.
What are the possible side effects of JELMYTO?
JELMYTO may cause serious side effects, including:- Swelling and narrowing of the tube that carries urine from the kidney to the bladder (ureteric obstruction). If you develop swelling and narrowing, and to protect your kidney from damage, your healthcare provider may recommend the placement of a small plastic tube (stent) in the ureter to help the kidney drain. Tell your healthcare provider right away if you develop side pain or fever during treatment with JELMYTO.
- Bone marrow problems. JELMYTO can affect your bone marrow and can cause a decrease in your white blood cell, red blood cell, and platelet counts. Your healthcare provider will do blood tests prior to each treatment to check your blood cell counts during treatment with JELMYTO. Your healthcare provider may need to temporarily or permanently stop JELMYTO if you develop bone marrow problems during treatment with JELMYTO.
- side pain
- urinary tract infection
- blood in your urine
- kidney problems
- tiredness
- nausea
- stomach pain
- trouble with urination
- vomiting
- low red blood cell count
- frequent urination
- itching
- chills
- fever
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You can also report side effects to UroGen Pharma at 1-855-987-6436.General information about JELMYTO.
Medicines are sometimes prescribed for purposes other than those listed in this Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about JELMYTO that is written for healthcare professionals.What are the ingredients of JELMYTO?
Active ingredient: mitomycin
Inactive ingredients: hydroxypropyl methylcellulose, mannitol, poloxamer, polyethylene glycol, and water for injection
Distributed by:
UroGen Pharma, Inc.
Princeton, NJ 08540
JELMYTO™ and UroGen® are trademarks of UroGen Pharma, Ltd.
U.S. Patent Nos. 9,040,074 and 9,950,069
Copyright© 2020 UroGen Pharma, Inc.
All rights reserved.
JEL-PPI-001
For more information go to www.JELMYTO.com or call 1-855-987-6436. - are pregnant or plan to become pregnant. JELMYTO can harm your unborn baby. You should not become pregnant during treatment with JELMYTO. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with JELMYTO.
-
INSTRUCTIONS FOR PHARMACY (IFP)JELMYTO™ (jel-MYE-toe)(mitomycin) for pyelocalyceal solution
Purpose of this Instructions for Pharmacy
This Instructions for Pharmacy contains information on how to prepare JELMYTO using pharmacy supplies and the UroGen Pharma Chilling Block.
Intended Use of JELMYTO
JELMYTO (mitomycin) for pyelocalyceal solution is indicated for the treatment of adult patients with low-grade Upper Tract Urothelial Cancer (LG-UTUC).
Important Information You Need to Know Before Reconstituting JELMYTO
Once reconstituted with sterile hydrogel, JELMYTO will appear as a semisolid gel. Once chilled, JELMYTO will convert to a viscous liquid.
Reconstituted JELMYTO must be prepared under chilled conditions. A UroGen Pharma Chilling Block can be used for this purpose. JELMYTO cannot be prepared without the Chilling Block or other means of chilling.
Preparation of JELMYTO must be performed under aseptic conditions.
Storage Conditions and Handling
Instill the JELMYTO solution as soon as possible after reconstitution. If immediate instillation is not possible, store reconstituted JELMYTO at 20°C to 25°C (68°F to 77°F) for up to 8 hours. Protect from light.
JELMYTO is a cytotoxic anti-cancer drug. Procedures for Proper Handling and Disposal of anti-cancer drugs should be followed.
Supplies Needed
JELMYTO Single-Dose Carton containing:
Do not substitute any of these components.- 2 Vials of JELMYTO, 40 mg/vial
- 1 Vial Sterile Hydrogel, 20 mL/vial
- 1 JELMYTO Admixture Label
- JELMYTO Full Prescribing Information (PI)
- JELMYTO Instructions for Pharmacy (IFP)
- JELMYTO Instructions for Administration (IFA)


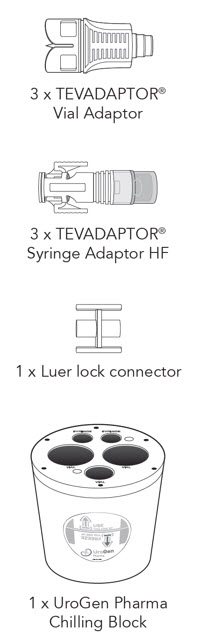
Pharmacy Supplies (Provided by Your Facility)
Do not substitute any of these components.- 3 × TEVADAPTOR® Vial Adaptors
- 3 × TEVADAPTOR® Syringe Adaptors HF
- 1 × Luer Lock connector
- 2 × 10 mL Luer Lock syringes
- 1 × 20 mL Luer Lock syringe
- 1 × 20-25G needle (for drawing up sterile water)
- 2 mL sterile water
- 70% Isopropyl alcohol or equivalent
- 1 × Light protective bag
- 1 × UroGen Pharma Chilling Block
Steps to Prepare JELMYTO Admixture
A. Freeze Chilling Block
- The day before preparation, put the Chilling Block in the freezer at -20°C to -12°C (-4°F to 10.4°F) upside down overnight.
Note: Please refer to the Chilling Block Instructions for Use for additional information.
B. Prepare Supplies
- Remove the Chilling Block from the freezer.
- Disinfect the Chilling Block by spraying it with 70% Isopropyl alcohol or equivalent. Allow it to air dry, and then place it upright inside the hood or isolator.
- Wait 20 minutes before continuing.
- Connect vial adaptors to all three vials.
- Connect a syringe adaptor to one of the 10 mL syringes.
- Connect a syringe adaptor to the 20 mL syringe.
- Place the three vials, the 10 mL syringe, and the 20 mL syringe into the Chilling Block for at least 10 minutes.
- While the vials and syringes are in the Chilling Block, withdraw 2 mL of sterile water into the other 10 mL syringe and set aside for later use.
C. Create Pre-Wetting Solution (PWS)
- Slowly fill the chilled 20 mL syringe with 14 mL of sterile hydrogel.
- Recap the chilled 20 mL syringe and place it in the Chilling Block.
- Slowly fill the chilled 10 mL syringe with 4 mL of sterile hydrogel.
- Discard the unused portion of sterile hydrogel.
- Replace the needle on 2 mL sterile water syringe with the Luer Lock connector.
- Remove the syringe adaptor from the 4 mL sterile hydrogel syringe.
- Connect the 4 mL sterile hydrogel syringe to the other side of the Luer Lock connector on the 2 mL sterile water syringe.
- Gently mix the sterile water with the sterile hydrogel by pushing the plungers back and forth at least 25 times to create the "pre-wetting solution" (PWS).
- Transfer the 6 mL PWS into one of the syringes.
- Replace the Luer Lock connector on the 6 mL PWS syringe with a new syringe adaptor.
- Place the 6 mL PWS syringe in the Chilling Block.
D. Mix the Admixture
- Remove both JELMYTO vials from the Chilling Block.
- Gently tap the bottom of each vial on the table to ensure all the mitomycin powder is at the bottom of the vials.
- Remove the chilled 6 mL PWS syringe from the Chilling Block.
- Inject 3 mL of PWS into each JELMYTO vial.
Note: To ensure accurate dosing, the contents of each vial must be the same. - Discard the empty PWS syringe.
- Gently swirl each JELMYTO vial upright at least 15 times, ensuring all powder and admixture is contained at the bottom of the vial.
Note: Do not invert or shake the vials. - Immediately remove the chilled 14 mL sterile hydrogel syringe from the Chilling Block.
- Immediately inject 7 mL of sterile hydrogel into each JELMYTO vial.
Note: To ensure accurate dosing, the contents of each vial must be the same. - Gently swirl each JELMYTO vial upright at least 15 times, ensuring all admixture is contained at the bottom of the vial.
Note: Do not invert or shake the vials. - Recap and place the 20 mL syringe in the Chilling Block.
- Mix the JELMYTO admixture vials:
- Recap and place both JELMYTO vials in the Chilling Block for five minutes.
- Remove both vials and vigorously swirl them upright at least 15 times, ensuring all admixture is contained at the bottom of the vials.
- Place both vials back in the Chilling Block.
- Repeat these steps every five minutes for a total of 30 minutes.
E. Prepare Admixture Vial
- Remove one JELMYTO vial from the Chilling Block.
- Vigorously swirl the vial upright at least 15 times.
Note: Do not invert or shake the vial. - Using the chilled 20 mL syringe, slowly withdraw 7 mL of admixture from the vial.
Note: If you are having difficulty withdrawing the admixture, place the components back in the Chilling Block until the admixture liquifies again. - Discard the empty JELMYTO vial.
- Remove the remaining JELMYTO vial from the Chilling Block.
- Inject the contents of the 20 mL syringe into that vial. Now all the admixture is contained in one vial.
- Recap the vial adaptor.
- Vigorously swirl the vial upright at least 15 times.
Note: Do not invert or shake the vial. - Your JELMYTO admixture vial is now complete.
F. Dispense Admixture Vial
- Write the "Discard after" date and time on the admixture label and apply to the prepared JELMYTO admixture vial.
Note: The "Discard after" date and time is 8 hours from the completion of the preparation at room temperature. - Place the following items in a light-protective bag:
- JELMYTO admixture vial
- JELMYTO Instructions for Administration
- Transport to the treatment facility.
 Important to Remember
Important to Remember
- All components must be kept cold during the preparation process by placing them in the Chilling Block when they are not in use.
- If you have difficulty pushing the solution or withdrawing the solution at any time, put the components back in the Chilling Block until the product liquifies.
Frequently Asked Questions
How do I connect the vial adaptor?
Place the vial adaptor over the vial and press down firmly. You will hear a snap which confirms successful attachment.
How do I connect the syringe adaptor?
Screw the Luer Lock end of the syringe adaptor onto the syringe hub until it is finger tight.
How do I connect the syringe adaptor to the vial adaptor?
Place the syringe adaptor over the vial adaptor and align the tabs, then press down firmly. You will hear a snap which confirms successful attachment.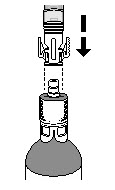
How do I disconnect the syringe adaptor from the vial adaptor?
Pinch the tabs on the syringe adaptor to separate it from the vial adaptor.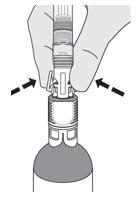
Do I need to put the caps on the syringes and vials before placing them back into the chilling block?
YES, the chilling block inserts are not sterile. It is important to maintain aseptic technique.Do I have to keep the vials upright when mixing? If so, why?
YES, the vials should be upright during all mixing to ensure the contents are fully mixed at the bottom of the vial.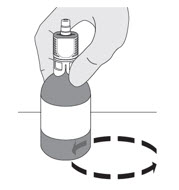
I am having difficulty working with the drug product. It seems to be solidifying.
If you are having difficulty pushing the solution or withdrawing the solution, place the components back into the Chilling Block until the product liquifies.This "Instructions for Pharmacy" has been approved by the U.S. Food and Drug Administration.
TEVADAPTOR® is a registered trademark of Teva Medical Ltd.
Distributed by:
UroGen Pharma, Inc.
Princeton, NJ 08540www.JELMYTO.com
JEL-IFP-001
-
INSTRUCTIONS FOR ADMINISTRATION (IFA)JELMYTO™ (jel-MYE-toe)(mitomycin) for pyelocalyceal solutionFor Pyelocalyceal Instillation Only

Read and follow this Instructions for Administration prior to each JELMYTO instillation. Purpose of this Instructions for Administration
This Instructions for Administration contains information on how to instill JELMYTO using the reconstituted JELMYTO vial which you received from the pharmacy and the devices listed under Supplies Needed, obtained by your facility.
Intended Use of JELMYTO
JELMYTO (mitomycin) for pyelocalyceal solution is indicated for the treatment of adult patients with low-grade Upper Tract Urothelial Cancer (LG-UTUC).
Important Information You Need to Know Before Instilling JELMYTO
JELMYTO must be reconstituted by a healthcare professional prior to instillation. Reconstituted JELMYTO will appear as a semisolid gel. Once chilled, JELMYTO will convert to a viscous liquid for instillation.
Once instilled into the patient's pyelocalyceal system, JELMYTO will fill and conform to the cavity and become a gel, thereby exposing the tissue to mitomycin over a prolonged period of time.
JELMYTO is viscous, even when it is a liquid in a chilled state. Therefore, you will need a Uroject12 Syringe Lever to instill JELMYTO into the patient. You cannot instill JELMYTO without the Uroject12 device.
Reconstituted JELMYTO should be instilled as soon as possible after reconstitution. If immediate instillation is not possible, store reconstituted JELMYTO at 20°C to 25°C (68°F to 77°F) for up to 8 hours. Protect from light.
When ready to instill, chill JELMYTO to 27° F to 41°F (-3°C to 5°C) for at least 10 minutes, but no longer than one hour, to revert it to a liquid form. See the Steps A through E for complete administration instructions.
JELMYTO is a cytotoxic anti-cancer drug. Procedures for Proper Handling and Disposal of anti-cancer drugs should be followed.
Supplies Needed
One vial of reconstituted JELMYTO
(prepared and provided by the pharmacy)
JELMYTO vial Ancillary Supplies
(to be provided by your facility):

Do not substitute any of these components. - TEVADAPTOR® Syringe Adaptor HF
- COP MEDALLION® 20 mL Luer Lock syringe
- Ureteral Catheter with molded Luer lock port (5 Fr or 7 Fr)
- Uroject12 Syringe Lever
Note: The Uroject12 Syringe Lever is a multi-use device and must be sterilized or disinfected before use. Please follow the sterilization or disinfection instructions detailed in the Uroject12 Syringe Lever Instructions for Use. - An ice bath to chill the vial of JELMYTO prior to instillation.

TEVADAPTOR® Syringe Adaptor HF COP MEDALLION® 20 mL Luer Lock syringe Ureteral Catheter with molded Luer lock port 

Uroject12 Syringe Lever Ice Bath Instillation Instructions
- A.
Measure the Kidney Volume
The Instillation Volume will be equal to the patient's kidney volume. If the kidney volume is already known, proceed to Section B.
If the kidney volume is NOT known, or needs to be reassessed, use the following steps:- Perform a retrograde pyelogram using diluted contrast (50%) so that the entire renal pelvis and calyces are observed and contrast starts to flow below the ureteropelvic junction (UPJ).
- Record the volume of contrast injected at this point.
- Allow the contrast to drain from the kidney. This may take about five minutes.
Note: Do not withdraw contrast back into the syringe. - Repeat Steps 1 through 3 two more times, for a total of three measurements to improve accuracy.
- Average the three volume measurements and round to the nearest whole number. This is the patient's kidney volume.
- B. Select the Instillation Volume
Select the kidney volume OR 15 mL, whichever is lower, as the Instillation Volume.
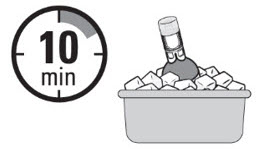
Note: Maximum Instillation Volume is 15 mL. Record this volume for future instillations.- C. Chill the JELMYTO
- Place the JELMYTO vial in the ice bath for at least 10 minutes, but no longer than one hour.
- After at least 10 minutes, advance the ureteral catheter over the guidewire towards the target anatomy in the pyelocalyceal system.
- D.
Prepare the Administration Syringe
Once the vial is removed from the ice bath, you have 4 minutes to draw JELMYTO into the administration syringe before it solidifies.
After 4 minutes, recap and place the components back in the ice bath for no more than 15 minutes to liquify the JELMYTO.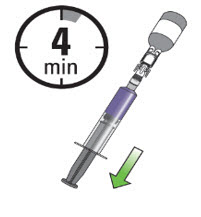
- Remove the JELMYTO vial from the ice bath and dry it off.
- Swirl the vial upright to ensure that JELMYTO is uniformly mixed.
- Connect the syringe adaptor to the 20 mL Luer Lock syringe. This will be the administration syringe.
- Slowly withdraw the calculated Instillation Volume of JELMYTO into the administration syringe.
- Press the "Clutch" button on the Uroject12 and pull the knob out.
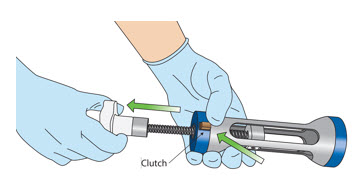
- Insert the administration syringe into the Uroject12 Syringe Lever and rotate it clockwise until it locks in place.
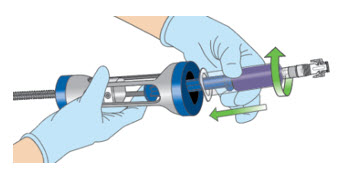
- While pressing the "Clutch" button, advance the lever to just above the administration syringe's plunger.
Immediately proceed with the instillation steps in Section E to avoid JELMYTO solidification.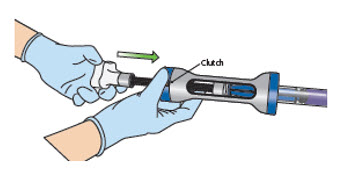
- E.
Instill JELMYTO
- Remove the syringe adaptor from the administration syringe.
Note: If JELMYTO gets on to the syringe tip or the catheter's Luer Lock port, wipe it off immediately with sterile gauze so it does not solidify and prevent a secure connection. (Refer to Frequently Asked Questions below for further details.) - Connect the administration syringe to the ureteral catheter's Luer Lock port, by rotating the syringe only.
- Using fluoroscopy, ensure the ureteral catheter is in the desired anatomical position.
- Gradually instill the JELMYTO into the patient by turning the knob at a rate of 1-2 seconds per stroke.
The entire syringe must be emptied within one minute.
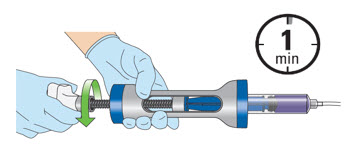
- Remove the ureteral catheter from the urinary tract.
- Remove the administration syringe from the Uroject12 by rotating the syringe barrel counter-clockwise.
- Discard the administration ancillaries according to your facility's disposal procedures.
- Send the Uroject12 to be reprocessed according to your facility's procedures and the Uroject12 Syringe Lever Instructions for Use.
- Remove the syringe adaptor from the administration syringe.
Frequently Asked Questions
How do I connect the syringe adaptor?
Screw the Luer Lock end of the syringe adaptor onto the syringe hub until it is finger tight.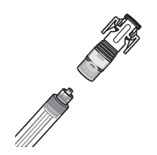
How do I connect the syringe adaptor to the vial adaptor?
Place the syringe adaptor over the vial adaptor and align the tabs; then press down firmly. You will hear a snap which confirms successful attachment.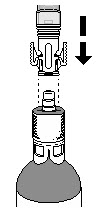
How do I disconnect the syringe adaptor from the vial adaptor?
Pinch the tabs on the syringe adaptor to separate it from the vial adaptor.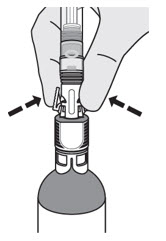
I am having a hard time working with reconstituted JELMYTO. It seems to be solidifying.
If you are having difficulty pushing or withdrawing JELMYTO, recap and place the components back into the ice bath until JELMYTO liquifies.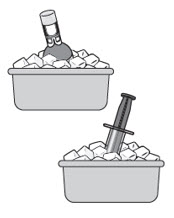
What should I do if there is JELMYTO spillage from the syringe tip?
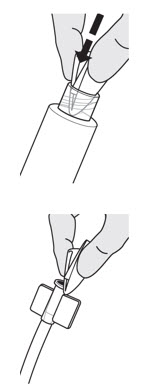
You will need to clean JELMYTO off the syringe tip and the Luer Lock port of the catheter to ensure the two components can connect securely.- To clean the syringe tip, fold a sterile gauze pad in half twice, and push it into the inside of the Luer Lock port to wipe any JELMYTO off the threads.
- To clean the Luer Lock port on the catheter, use a sterile gauze pad to wipe off any JELMYTO.
- Verify that the connectors are clean to ensure a secure connection between the syringe and the catheter.
This "Instructions for Administration" has been approved by the U.S. Food and Drug Administration.
Distributed by:
UroGen Pharma, Inc.
Princeton, NJ 08540www.JELMYTO.com
TEVADAPTOR® is a registered trademark of Teva Medical Ltd.
MEDALLION® is a registered trademark of Merit Medical Systems, Inc.
JEL-IFU-001
-
PRINCIPAL DISPLAY PANEL - Kit Carton
NDC: 72493-103-03
Jelmyto™
(mitomycin) for pyelocalyceal solutionAttention Pharmacist: Reconstitution
is required prior to dispensing.See the Instructions for Pharmacy
before proceeding.SINGLE-DOSE CARTON
Warning: For Pyelocalyceal Use Only
Rx OnlyContents of Carton:
- 2 Vials of JELMYTO™ (mitomycin) for pyelocalyceal solution, 40 mg per Vial
- 1 Vial Sterile Hydrogel, 20 mL per Vial
- 1 Admixture Label
- Full Prescribing Information
- Instructions for Pharmacy
- Instructions for Administration
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F).
Avoid excessive heat over 40°C (104°F). Protect from light.Reconstituted JELMYTO should be instilled as soon as possible after reconstitution. If immediate instillation
is not possible, store reconstituted JELMYTO at 20°C to 25°C (68°F to 77°F) for up to 8 hours.
Protect reconstituted JELMYTO from light.When ready to instill, chill JELMYTO to -3°C to 5°C (27°F to 41°F) for at least 10 minutes,
but no longer than one hour, to revert it to a liquid form.UroGen®
Pharma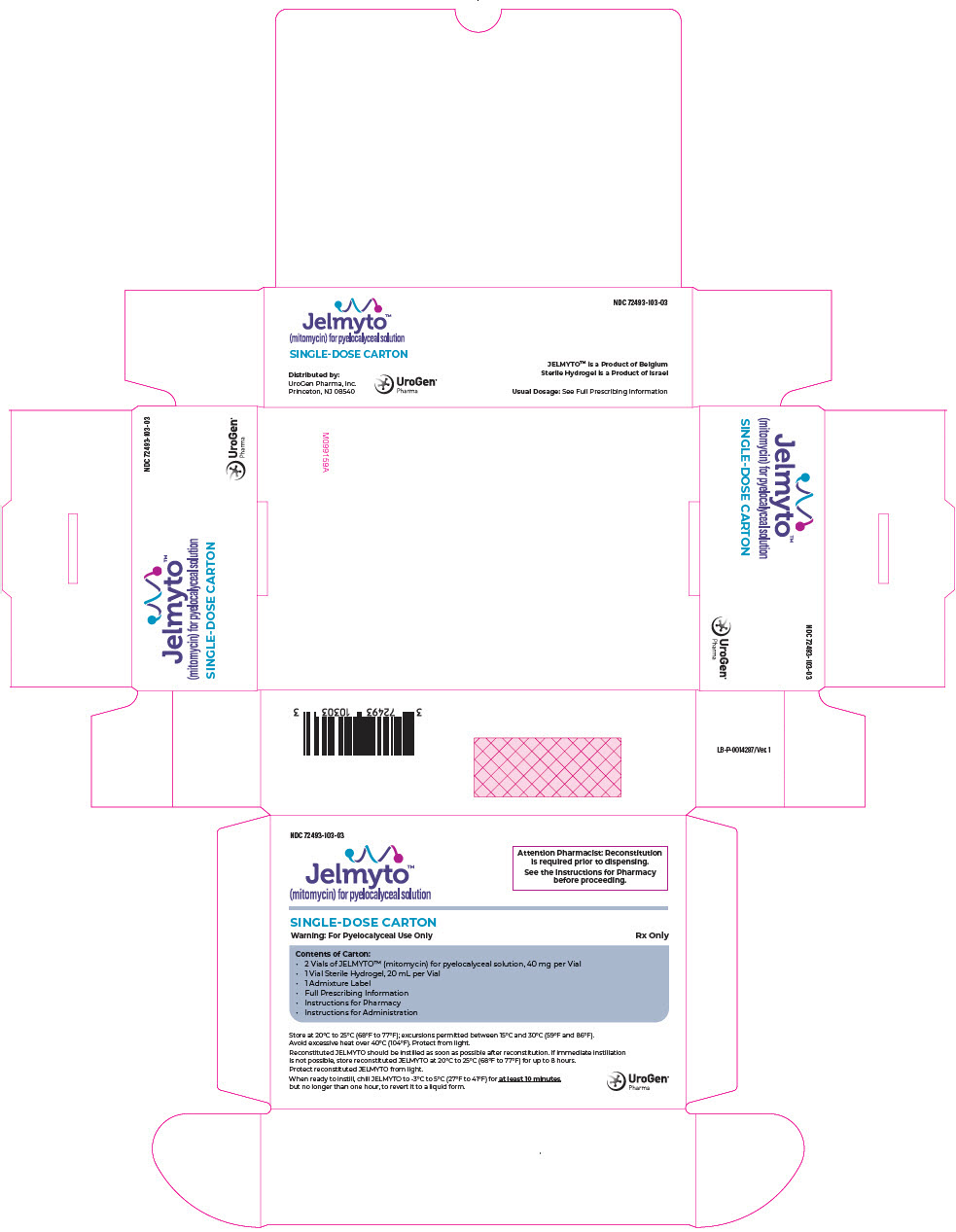
-
PRINCIPAL DISPLAY PANEL - 40 mg Vial Label
NDC: 72493-101-40
Single-Dose Vial
SterileJelmyto™
(mitomycin) for pyelocalyceal solution40 mg per Vial
See Instructions for Pharmacy for preparation instructions
Must be Reconstituted with Sterile Hydrogel Before Use
Warning: For Pyelocalyceal Use OnlyStore at 20°C to 25°C (68°F to 77°F); excursions permitted
between 15°C and 30°C (59°F and 86°F). Avoid excessive heat
over 40°C (104°F). Protect from light.
Rx Only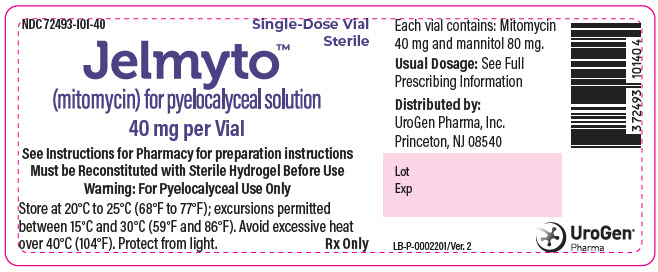
-
PRINCIPAL DISPLAY PANEL - 20 mL Vial Label
NDC: 72493-102-20
Single-Dose VialSterile Hydrogel
For use in preparation of JELMYTO™
(mitomycin) for pyelocalyceal solutionNot for Direct Administration
See Instructions for Pharmacy for
preparation instructionsStore at 20°C to 25°C (68°F to 77°F); excursions
permitted between 15°C and 30°C (59°F and 86°F).
Avoid excessive heat over 40°C (104°F).Rx Only
20 mL per Vial
-
INGREDIENTS AND APPEARANCE
JELMYTO
mitomycin kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72493-103 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72493-103-03 1 in 1 CARTON 05/01/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 VIAL, SINGLE-DOSE 2 Part 2 1 VIAL, SINGLE-DOSE 20 mL Part 1 of 2 JELMYTO
mitomycin powder, for solutionProduct Information Item Code (Source) NDC: 72493-101 Route of Administration URETERAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Mitomycin (UNII: 50SG953SK6) (Mitomycin - UNII:50SG953SK6) Mitomycin 40 mg Inactive Ingredients Ingredient Name Strength Mannitol (UNII: 3OWL53L36A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72493-101-40 1 in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211728 05/01/2020 Part 2 of 2 JELMYTO
sterile hydrogel gelProduct Information Item Code (Source) NDC: 72493-102 Route of Administration URETERAL Inactive Ingredients Ingredient Name Strength POLOXAMER 407 (UNII: TUF2IVW3M2) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72493-102-20 20 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211728 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211728 05/01/2020 Labeler - UroGen Pharma, Inc. (081130487) Registrant - UroGen Pharma, Ltd. (534155213)
Trademark Results [JELMYTO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 JELMYTO 88204510 not registered Live/Pending |
UROGEN PHARMA LTD. 2018-11-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.