endure by CVS Pharmacy / Natureplex LLC ENDURE- lidocaine spray
endure by
Drug Labeling and Warnings
endure by is a Otc medication manufactured, distributed, or labeled by CVS Pharmacy, Natureplex LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- Uses
- Warnings
- Do not use
- Ask a doctor before use
- Ask a doctor or pharmacist before use if you are
-
When using this product
- do not get into eyes or nostrils
- do not inhale
- do not exceed a maximum of 20 sprays in 24 hours
- always use the minimum amount effective for you
- allow 5-15 minutes to dry prior to intercourse
Can be used for sexual intercourse and sex play when applied in accordance with the usage instructions. Only use in accordance with the instructions, seek medical attention immediately in case of overdose.
This product is not compatible with condoms.
- Stop use and ask a doctor if
- Keep out of reach of children
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Instructions For Use
Male Genital Desensitizer
For Prolonged Pleasure
SPRAY FOR MEN
Lidocaine (approximately 10mg per spray)
Active Ingredient:
Lidocaine USP 8.4% (approximately 10 mg per spray)
Purpose: Male Genital Desensitizer
Uses:
- Helps in the prevention of premature ejaculation
- Helps in temporarily prolonging the time until ejaculation
Warnings
- For external use only
- Avoid contact with the eyes
Allergy alert: Do not use this product if you or your partner are allergic (sensitive) to local anesthetics or any of the other listed ingredients
Do not use on broken or inflamed skin
Ask a doctor before use
- if your partner is pregnant or breast-feeding
- if you have or have had liver or kidney problems
Ask a doctor or pharmacist before use if you are already taking prescribed drugs
When using this product:
- Do not get into eyes or nostrils
- Do not inhale
- Do not exceed a maximum of 20 sprays in 24 hours
- Always use the minimum amount effective for you
- Allow 5-15 minutes to dry prior to intercourse
Can be used for sexual intercourse and sex play when applied in accordance with the usage instructions. Only use in accordance with the instructions, seek medical attention immediately in case of overdose.
This product is not compatible with condoms.
Stop use and ask a doctor if
- this product, used as directed, does not provide relief
- you or your partner develop a rash or irritation, such as burning or itching
- symptoms persist. Premature ejaculation may be due to a condition requiring medical supervision.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away (1-800-222-1222).
Directions
- Use only as directed
- Apply 3 or more sprays, not to exceed 10, to the head and shaft of the penis before intercourse, or use as directed by a doctor
- Wash product off after intercourse
- Please read the instructions for use and advice inside this pack carefully to achieve best results
Other information
- Store at 20-25°C (68-77°F)
- The bottle is child-resistant. Please refer to Opening the Child Resistant Closure section on the reverse side of this leaflet for instructions for opening.
Inactive ingredients:
Diethylene Glycol Monoethyl Ether, Fragrance, Isopropyl Myristate, Stearic Acid, Vitamin E
Questions? 1-800-719-9260
2Q000 00 J1
This product is an endurance enhancer which temporarily prolongs the time until ejaculation so he can have more control and prolong pleasure!
Opening the Child Resistant Closure
The spray bottle is fitted with a child resistant lock. Follow the below steps to open the lock and press the pump several times. On first use, it may take up to a few presses to release the product. When using the product next time, you will dispense the right amount quicker.
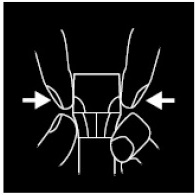
Place index finger and thumb on ribbed collar. Hold collar firmly. Using the opposite hand, place index finger and thumb on ribbed portion of the cap and squeeze.
As you squeeze the cap and hold the collar – turn cap counter-clockwise to remove.
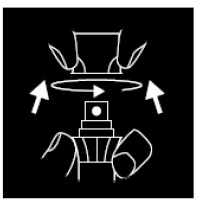
Pump to release product. Secure cap after use.
Applying this Product
Everyone is different. So, for you, maybe it’s 3 sprays or maybe it’s more. It takes time to understand the right amount for you and how to get the most from this product. For the first time, try 3 sprays.
Approximately 5-15 minutes prior to intercourse,
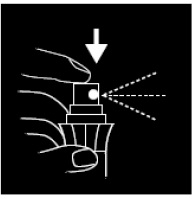
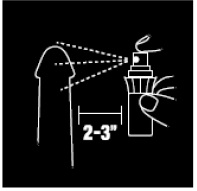
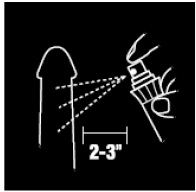
- 1. Hold bottle upright 2” to 3” from penis.
- 2. Apply 3 or more sprays, not to exceed 10, to the head and shaft of the penis before intercourse, or use as directed by a doctor.
- 3. Rub in as required, and then wipe off any excess with a soft, damp cloth prior to intercourse.
- 4. Wash product off after intercourse.
Tip 1 – While you are waiting for the spray to take effect, there’s no better time to use your foreplay skills to tease your partner and increase their desire so they are far more likely to climax.
Tip 2 – Take control of how long you want to last to get it right for you and your partner. If you didn’t last as long as desired for the previous time, try an extra spray until both of you are happy (not to exceed 10 sprays unless advised by your doctor). Try reducing the number of sprays if you or your partner experience any reduction in sensation. Keep experimenting with number of sprays and waiting time, as it can take a few tries to get the right level for you both.
Distributed By
Perrigo®
Allegan, MI 49010
perrigo.com
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ENDURE
lidocaine sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69842-663 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) STEARIC ACID (UNII: 4ELV7Z65AP) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69842-663-01 1 in 1 CARTON 04/18/2019 1 6.5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part348 04/18/2019 Labeler - CVS Pharmacy (062312574)
Trademark Results [endure]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ENDURE 98782656 not registered Live/Pending |
Mighty Squirrel Brewing Co., LLC 2024-10-02 |
 ENDURE 98759156 not registered Live/Pending |
LUBRICATION ENGINEERS, INC. 2024-09-19 |
 ENDURE 98746859 not registered Live/Pending |
Ecolab USA Inc. 2024-09-12 |
 ENDURE 98651962 not registered Live/Pending |
Hangzhou Haosheng Mechanical and Electrical Industry and Trade Co., Ltd. 2024-07-17 |
 ENDURE 98651940 not registered Live/Pending |
Hangzhou Haosheng Mechanical and Electrical Industry and Trade Co., Ltd. 2024-07-17 |
 ENDURE 98647832 not registered Live/Pending |
Berry-Koert, Michelle 2024-07-15 |
 ENDURE 98478207 not registered Live/Pending |
FIRST CHOICE SOURCING SOLUTIONS, LLC 2024-04-01 |
 ENDURE 98445881 not registered Live/Pending |
CALLAN FAMILY OFFICE, LLC 2024-03-12 |
 ENDURE 98426808 not registered Live/Pending |
MetroWall, LLC 2024-02-29 |
 ENDURE 98223995 not registered Live/Pending |
EPC IP Co LLC 2023-10-14 |
 ENDURE 98218478 not registered Live/Pending |
THE EVEREST PROJECT LLC 2023-10-11 |
 ENDURE 97214691 not registered Live/Pending |
Endure Industries, Inc. 2022-01-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.