These highlights do not include all the information needed to use ULESFIA® safely and effectively. See full prescribing information for ULESFIA® . ULESFIA® (benzyl alcohol) lotion, for topical use Initial U.S. Approval: 2009
Ulesfia by
Drug Labeling and Warnings
Ulesfia by is a Prescription medication manufactured, distributed, or labeled by Lachlan Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ULESFIA - benzyl alcohol lotion
Lachlan Pharmaceuticals
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ULESFIA® safely and effectively. See full prescribing information for ULESFIA® .
ULESFIA® (benzyl alcohol) lotion, for topical use Initial U.S. Approval: 2009 INDICATIONS AND USAGEDOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSLotion, 5% (3) CONTRAINDICATIONSNone. (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions (> 1% and more common than with placebo): ocular irritation, application site irritation, and application site anesthesia and hypoesthesia. (6)
DRUG INTERACTIONSDrug interaction studies were not conducted. (7) USE IN SPECIFIC POPULATIONSPediatric Use: Safety in pediatric patients under six months of age has not been established. (8.4) See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 6/2015 |
|||||||||||||||||||||||||||||||||
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Indication
ULESFIA® Lotion is indicated for the topical treatment of head lice infestation in patients 6 months of age and older.
1.3 Adjunctive Measures
ULESFIA® Lotion should be used in the context of an overall lice management program:
- Wash (in hot water) or dry-clean all recently worn clothing, hats, used bedding, and towels.
- Wash personal care items such as combs, brushes and hair clips in hot water.
- A fine-tooth comb or special nit comb may be used to remove dead lice and nits.
2 DOSAGE AND ADMINISTRATION
ULESFIA® Lotion is not for oral, ophthalmic, or intravaginal use.
Using the guidelines in Table 1, apply sufficient ULESFIA® Lotion to dry hair to completely saturate the scalp and hair; leave on for 10 minutes, then thoroughly rinse off with water. Repeat application after 7 days. Avoid contact with eyes.
| Hair Length | Amount of ULESFIA® Lotion per Application |
||
| Ounces
| 8 oz bottle Size
|
||
| Short | 0-2 inches | 4-6 oz | ½-¾ bottle |
| 2-4 inches | 6-8 oz | ¾-1 bottle |
|
| Medium | 4-8 inches | 8-12 oz | 1-1½ bottles |
| 8-16 inches | 12-24 oz | 1½-3 bottles |
|
| Long | 16-22 inches | 24-32 oz | 3-4 bottles |
| Over 22 inches | 32-48 oz | 4-6 bottles |
|
3 DOSAGE FORMS AND STRENGTHS
ULESFIA® Lotion is a white topical lotion containing benzyl alcohol, 5% (50 mg/g of lotion).
5 WARNINGS AND PRECAUTIONS
5.1 Neonatal Toxicity
Intravenous administration of products containing benzyl alcohol has been associated with neonatal gasping syndrome consisting of severe metabolic acidosis, gasping respirations, progressive hypotension, seizures, central nervous system depression, intraventricular hemorrhage, and death in preterm, low birth weight infants. Neonates (i.e. patients less than 1 month of age or preterm infants with a corrected age of less than 44 weeks) could be at risk for gasping syndrome if treated with ULESFIA® Lotion [see Use in Specific Populations (8.4)].
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The rates of adverse reactions below were derived from two randomized, multi-center, vehicle-controlled clinical trials and one open-label study in subjects with head lice infestation.
Skin, scalp, and ocular irritation were monitored in the clinical trials. All subjects were queried about the presence of skin and scalp symptoms; the results are presented in Table 2.
| Event | ULESFIA®
Lotion | Vehicle |
| Application site Irritation | 2% (11/478) | 1% (2/336) |
| Application site anesthesia & hypoesthesia | 2% (10/478) | 0% (0/336) |
| Pain | 1% (5/478) | 0% (1/336) |
The subset of subjects who did not have pruritus, erythema, edema or pyoderma of skin and scalp, or ocular irritation prior to treatment were assessed for these signs and symptoms after treatment; the results are presented in Table 3.
| Signs/Symptoms | ULESFIA®
Lotion | Vehicle |
| Pruritus | 12% (14/116) | 4% (3/67) |
| Erythema | 10% (32/309) | 9% (19/217) |
| Pyoderma | 7% (22/308) | 4% (10/230) |
| Ocular irritation | 6% (26/428) | 1% (3/313) |
Other less common reactions (less than 1% but more than 0.1%) were, in decreasing order of incidence: application site dryness, application site excoriation, paraesthesia, application site dermatitis, excoriation, thermal burn, dandruff, erythema, rash, and skin exfoliation.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
There are no adequate and well-controlled studies with topical benzyl alcohol in pregnant women. Reproduction studies conducted in rats and rabbits were negative. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
No comparisons of animal exposure with human exposure are provided in this labeling due to the low systemic exposure noted in the clinical pharmacokinetic study [see Clinical Pharmacology (12.3)] which did not allow for the determination of human AUC values that could be used for this calculation.
Pregnant rats were dosed with benzyl alcohol via subcutaneous injection at 100, 250, and 500 mg/kg/day. No teratogenic effects were noted at any dose. Maternal toxicity and decreased fetal weight occurred at 500 mg/kg/day. When pregnant rabbits received subcutaneous injections of benzyl alcohol at 100, 250, and 400 mg/kg/day, there were no teratogenic effects in offspring at any dose. In rabbits, maternal toxicity occurred at the two higher doses and was associated with decreased fetal weight at the highest dose.
8.3 Nursing Mothers
It is not known whether benzyl alcohol is excreted into human milk. Because some systemic absorption of topical benzyl alcohol may occur and because many drugs are excreted in human milk, caution should be exercised when ULESFIA® Lotion is administered to a nursing woman.
8.4 Pediatric Use
The safety and effectiveness of ULESFIA® Lotion was evaluated in two multicenter, randomized, double-blind, vehicle-controlled studies which were conducted in 628 subjects 6 months of age and older with active head lice infestation [see Clinical Studies (14)].
Rates of adverse events in younger children (6 months to 12 years) were similar to those of older children and adults.
Safety in pediatric patients below the age of 6 months has not been established. ULESFIA® Lotion is not recommended in pediatric patients under six months of age because of the potential for increased systemic absorption due to a high ratio of skin surface area to body mass and the potential for an immature skin barrier.
Neonates could be at risk for gasping syndrome if treated with ULESFIA® Lotion [see Warnings and Precautions (5.1)].
Intravenous administration of products containing benzyl alcohol has been associated with neonatal gasping syndrome. The gasping syndrome (characterized by central nervous depression, metabolic acidosis, gasping respirations, and high levels of benzyl alcohol and its metabolites found in the blood and urine) has been associated with benzyl alcohol dosages > 99 mg/kg/day in preterm neonates. Additional symptoms may include gradual neurological deterioration, seizures, intracranial hemorrhage, hemotologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. Although expected systemic exposure of benzyl alcohol from proper use of ULESFIA® Lotion is substantially lower than those reported in association with the gasping syndrome, the minimum amount of benzyl alcohol at which toxicity may occur is not known.
11 DESCRIPTION
ULESFIA® (benzyl alcohol) Lotion is supplied as a white topical lotion containing benzyl alcohol, 5%. Inactive ingredients in this formulation are water, mineral oil, sorbitan monooleate, polysorbate 80, carbomer 934P and trolamine.
The active ingredient, benzyl alcohol, is a clear, colorless liquid with a mild aromatic odor. Benzyl alcohol has a molecular mass of 108.14 g/mol. The molecular formula is C7H8O.
The chemical structure of benzyl alcohol is:
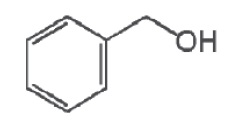
Benzyl Alcohol
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
In vitro studies of the effect of ULESFIA® Lotion on native, captured lice suggest that benzyl alcohol inhibits lice from closing their respiratory spiracles, allowing the vehicle to obstruct the spiracles and causing the lice to asphyxiate.
12.3 Pharmacokinetics
The absorption of benzyl alcohol from ULESFIA® Lotion was evaluated in 19 subjects with head lice infestation. Subjects were divided into two age groups: 6 months to 3 years and 4 to 11 years. ULESFIA® Lotion was applied for an exaggerated exposure period (3 times the normal exposure period). Benzyl alcohol was quantified in a single plasma sample in 4 out of 19 subjects (21%): three subjects in the 6 months to 3 years age group at 0.5 hour post-treatment (ranging from 1.97 to 2.99 mcg/mL) and one subject in the 4 to 11 year age group (1.63 mcg/mL) at 1 hour post-treatment out of a total of 102 samples analyzed.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate carcinogenic potential of ULESFIA® Lotion have not been conducted. No evidence of carcinogenic activity was noted for benzyl alcohol in 2 year oral carcinogenicity studies in rats (doses up to 400 mg/kg benzyl alcohol) or mice (doses up to 200 mg/kg benzyl alcohol) conducted by the National Toxicology Program.
Benzyl alcohol has produced mixed results in genetic testing. Benzyl alcohol was negative in the Ames test with and without metabolic activation, sex-linked recessive lethal assay, and a replicative DNA synthesis assay (conducted in male rats). Negative results were obtained in the mouse lymphoma assay with metabolic activation, but a positive response was noted in the mouse lymphoma assay without metabolic activation at a concentration producing a high level of cellular toxicity. Benzyl alcohol was positive in the Chinese hamster ovary chromosomal aberration assay with metabolic activation.
No fertility studies have been conducted with benzyl alcohol.
14 CLINICAL STUDIES
Two multicenter, randomized, double-blind, vehicle-controlled studies were conducted in 628 subjects 6 months of age and older with active head lice infestation. For the evaluation of efficacy, the youngest subject from each household was enrolled in the Primary Treatment Cohort with ULESFIA® Lotion or vehicle. Other infested household members were enrolled in a Secondary Treatment Cohort and received the same treatment as the youngest subjects. The Secondary Treatment Cohort was not included in the efficacy analysis, but was evaluated for all safety parameters.
In Study One, 125 Primary Treatment Cohort Subjects were randomized to ULESFIA® Lotion (N=63) and vehicle (N=62). Study Two enrolled 125 Primary Treatment Cohort Subjects: 64 randomized to ULESFIA® Lotion and 61 to vehicle. Treatment was applied two times separated by one week.
Efficacy was assessed as the proportion of subjects who were free of live lice 14 days after the final treatment. Subjects with live lice present at any time after first treatment were considered to be treatment failures. Table 4 contains the proportion of subjects who were free of live lice in each of the two trials.
| ULESFIA® LOTION
| Vehicle
|
|
| Study 1
| (N=63)
48 (76.2%) | (N=62)
3 (4.8%) |
| Study 2
| (N=64)
48 (75.0%) | (N=61)
16 (26.2%) |
16 HOW SUPPLIED/STORAGE AND HANDLING
ULESFIA® Lotion is a white topical lotion containing benzyl alcohol, 5% (50 mg/g of lotion)
● NDC: 69202-780-08 as an 8 fl oz (227 g) in a polypropylene bottle
● NDC: 69202-780-88 as a package containing two individual 8 fl oz (NDC: 69202-780-08) polypropylene bottles with a nit comb
Store at 20º C - 25º C (68º F - 77º F); excursions permitted to 15º C - 30º C (59º F - 86º F) [See USP controlled room temperature].
Do not freeze.
17 PATIENT COUNSELING INFORMATION
“See FDA-approved patient labeling (Patient Information)”
Inform the patient and caregiver of the following instructions:
- Apply ULESFIA® Lotion for 10 minutes as directed in the ULESFIA® Lotion Usage Guideline Table.
- Apply a second treatment 1 week (7 days) after the initial application.
- Use only on dry scalp and dry scalp hair.
- Avoid contact with eyes.
- ULESFIA® Lotion may cause eye irritation, skin irritation, and contact sensitization.
- Wash hands after application.
- Report any signs of irritation at the application site and any signs of adverse reactions.
- Keep out of reach of children.
Patient Information
ULESFIA®
(Yoo-Les-fee-ah)
(benzyl alcohol 5%)
Lotion
Read the Patient Information that comes with ULESFIA® Lotion before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your healthcare provider about your medical condition or treatment.
|
Note: For use on scalp hair and scalp only |
What is ULESFIA® Lotion?
ULESFIA® Lotion is a prescription medicine used to get rid of lice in scalp hair of children and adults.
It is not known if ULESFIA® Lotion is safe for children under 6 months of age or in people over age 60.
Once ULESFIA® Lotion is washed off, a fine-tooth comb may be used to remove treated lice and nits from the hair and scalp. All personal items exposed to the hair or lice should be washed in hot water or dry-cleaned. See "How do I stop the spread of lice?” at the end of this leaflet.
What should I tell my healthcare provider before I use Ulesfia® Lotion?
Tell your healthcare provider if your baby was born early so your healthcare provider can decide if your infant is old enough for ULESFIA® Lotion.
Before you use ULESFIA® Lotion, tell your healthcare provider if you:
- have any skin conditions or sensitivities
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if ULESFIA® Lotion can harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- are breastfeeding. It is not known if ULESFIA® Lotion passes into your breast milk or if it can harm your baby. You should choose to breastfeed or use ULESFIA® Lotion, but not both. Talk to your healthcare provider about other ways to feed your baby while using ULESFIA® Lotion.
How should I use ULESFIA® Lotion?
- Use ULESFIA® Lotion exactly as prescribed. Your healthcare provider will prescribe the treatment that is right for you. Do not change your treatment unless you talk to your healthcare provider.
- Use ULESFIA® Lotion in two applications that are one week apart. ULESFIA® Lotion gets rid of lice but does not get rid of lice eggs so a second application is needed one week (7 days) after the first application.
- ULESFIA® Lotion coats the lice on your scalp and scalp hair. It is important to use enough ULESFIA® Lotion to coat completely every single louse and to leave it on your scalp for the full 10 minutes. See the detailed Instructions for Use at the end of this leaflet.
- Because you need to completely cover all of the lice with ULESFIA® Lotion, you may need help in applying ULESFIA® Lotion to your scalp and hair. Make sure that you and anyone who helps you apply ULESFIA® Lotion reads and understands this leaflet and the Instructions for Use.
- Children need adult help in applying ULESFIA® Lotion.
- Do not get into eyes. If ULESFIA® Lotion gets in the eye, flush with water right away.
- Do not swallow ULESFIA® Lotion. If swallowed, call your healthcare provider right away.
- Wash your hands after you apply ULESFIA® Lotion.
What are the possible side effects of ULESFIA® Lotion?
People using ULESFIA® Lotion may have skin or eye:
- itching,
- redness,
- irritation. If skin or eye irritation happens, rinse with water right away, then call your healthcare provider or go to the emergency department.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the side effects of ULESFIA® Lotion. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ULESFIA® Lotion?
- Store ULESFIA® Lotion in a dry place at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze ULESFIA® Lotion.
Keep ULESFIA® Lotion and all medicines out of the reach of children.
What are the ingredients in ULESFIA® Lotion?
Active ingredient: benzyl alcohol, 5%
Inactive ingredients: purified water, mineral oil, sorbitan monooleate, polysorbate 80, carbomer 934P and trolamine
General Information about ULESFIA® Lotion
Medicines are sometimes prescribed for conditions other than those described in the Patient Information leaflet. Do not use ULESFIA® Lotion for any condition for which it was not prescribed by your healthcare provider. Do not give ULESFIA® Lotion to other people, even if they have the same symptoms as you. It may harm them.
This leaflet summarizes the most important information about ULESFIA® Lotion. If you would like more information, talk to your healthcare provider. You can also ask your healthcare provider for information about ULESFIA® Lotion that is written for healthcare professionals.
Instructions for Use
Apply the full amount of ULESFIA® Lotion prescribed by your healthcare provider for each of your 2 applications one week apart. ULESFIA® Lotion gets rid of lice but does not get rid of lice eggs so a second application is needed one week (7 days) after the first application.
ULESFIA® Lotion comes in a single 8 oz. bottle, and in a 2 pack containing two 8 oz. bottles with a lice comb. For different hair lengths, use the following as a guide for the amount of ULESFIA® Lotion you may need to cover the hair and scalp completely:
|
Hair Length |
Amount of ULESFIA® Lotion to apply each time |
|
8 oz. bottle |
|
|
Short- up to 2 inches |
up to ¾ the bottle |
|
Short- 2-4 inches |
up to 1 bottle |
|
Medium- 4-8 inches |
up to 1½ bottles |
|
Medium- 8-16 inches |
up to 3 bottles |
|
Long- 16-22 inches |
up to 4 bottles |
|
Long- 22 inches or more |
up to 6 bottles |
Step 1
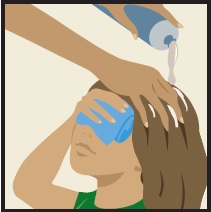
- Cover your face and eyes with a towel and keep your eyes closed tightly.
- Apply ULESFIA® Lotion directly to dry scalp and dry hair.
- ULESFIA® Lotion must cover your entire scalp and all scalp hair. Have an adult help you apply ULESFIA® Lotion.
Step 2

- Massage ULESFIA® Lotion into your hair and scalp.
- When your head is completely covered with enough ULESFIA® Lotion, dripping will happen. Protect your eyes and skin from these drips with your towel.

- Be sure to apply ULESFIA® Lotion to the area behind your ears.

- Be sure to apply ULESFIA® Lotion to the back of your neck.
If not enough ULESFIA® Lotion is used, some lice may escape treatment. It is important to use the full amount of ULESFIA® Lotion prescribed by your healthcare provider.

Correct application of ULESFIA® Lotion.
Step 3
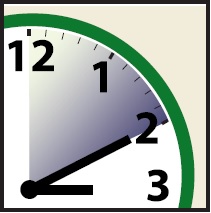
- Allow ULESFIA® Lotion to stay on your hair for 10 minutes. Use a timer or clock.
- Start timing after you have completely covered your hair and scalp with ULESFIA® Lotion .
Step 4

- After 10 minutes, completely rinse ULESFIA® Lotion from your hair and scalp with water.
- You or anyone who helps you apply ULESFIA® Lotion should wash your hands after application.
- You can shampoo your hair right after the application.
- A lice comb may be used to remove the dead lice after both applications.
Step 5
One week (7 days) after your first application, repeat the steps above to help get rid of lice that hatched from eggs.
How do I stop the spread of lice?
To help prevent the spread of lice from one person to another, here are some steps you can take.
- Avoid head-to-head contact at school, on the playground, in physical education classes, during sports activities, and while playing with other children.
- Do not share combs, brushes, hats, scarves, bandannas, ribbons, barrettes, hair bands, towels, helmets, or other hair-related personal items with anyone else, whether they have lice or not.
- Avoid sleepovers and slumber parties during lice outbreaks. Lice can live in bedding, pillows, and carpets that have recently been used by someone with lice.
- After finishing treatment with lice medicine, check everyone in your family for lice after one week. Be sure to talk to your healthcare provider about treatments for those who have lice.
- Machine-wash any bedding and clothing used by anyone having lice or thought to have been exposed to lice. Machine wash at high temperatures (150°F) and tumble in a hot dryer for 20 minutes.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Lachlan Pharmaceuticals
Dublin, Ireland
Manufactured by:
Contract Pharmaceuticals Limited
Mississauga, ON L5N 6L6
Canada
Revised: 06/2015
PI780-88.1
PRINCIPAL DISPLAY PANEL
NDC: 69202-780-88 Rx only
Ulesfia®
(benzyl alcohol)
Lotion, 5%
2-PACK
with lice comb
8 oz (227 g) per bottle
2 bottles in the carton
Unit for dispensing
| ULESFIA
benzyl alcohol lotion |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Lachlan Pharmaceuticals (985586938) |