BANAMINE- flunixin meglumine solution
Banamine by
Drug Labeling and Warnings
Banamine by is a Animal medication manufactured, distributed, or labeled by Merck Sharp & Dohme Corp., Vet Pharma Friesoythe GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- INFORMATION FOR OWNERS/CAREGIVERS
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
- VETERINARY INDICATIONS
-
DOSAGE & ADMINISTRATION
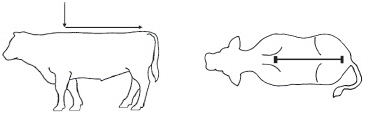
DOSAGE AND ADMINISTRATION: Apply only once at a dose of 3.3 mg flunixin per kg body weight (1.5 mg/lb; 3 mL per 100 lbs) topically in a narrow strip along the dorsal midline from the withers to the tailhead. Round all doses up to the nearest weight increment on the dosing chamber. Do not treat cattle if the hide is wet or may get wet in the six hours after dosing because effectiveness has not been evaluated under wet hide conditions.
Practice the Administration and Overfill Reduction Instructions a few times to become familiar with operating the package before dosing animals.
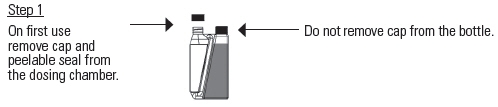
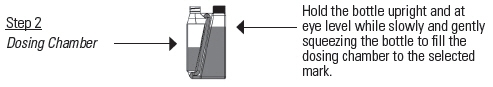
If the dosing chamber is overfilled follow the Overfill Reduction Instructions.
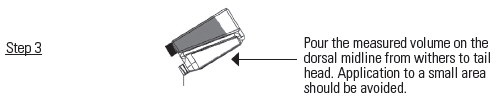
A small amount of liquid will remain on the walls of the chamber, but the chamber is calibrated to account for this.
OVERFILL REDUCTION INSTRUCTIONS
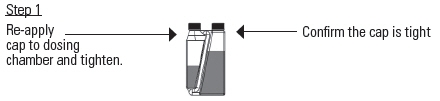
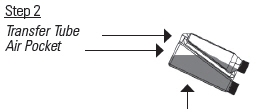
Tilt the bottle to allow an air pocket to form at the beginning of the transfer tube inside the bottle.
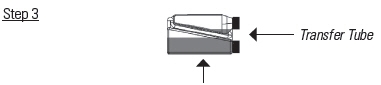
Hold the bottle horizontally to allow product to cover the end of the transfer tube inside the dosing chamber.
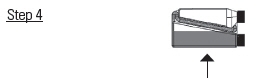
Squeeze and release the bottle repeatedly.
Product will return to the bottle through the transfer tube.Figure 1 – Recommended pour-on location
-
CONTRAINDICATIONS
CONTRAINDICATIONS: NSAIDs inhibit production of prostaglandins which are important in signaling the initiation of parturition. The use of flunixin can delay parturition and prolong labor which may increase the risk of stillbirth. Do not use Banamine Transdermal pour-on within 48 hours of expected parturition. Do not use in animals showing hypersensitivity to flunixin meglumine.
-
SAFE HANDLING WARNING
USER SAFETY WARNINGS: Not for use in humans. Keep out of reach of children. Flunixin transdermal solution is a potent non-steroidal anti-inflammatory drug (NSAID), and ingestion may cause gastrointestinal irritation and bleeding, kidney, and central nervous system effects.
This product has been shown to cause severe and potentially irreversible eye damage (conjunctivitis, iritis, and corneal opacity) and irritation to skin in laboratory animals. Users should wear suitable eye protection (face shields, safety glasses, or goggles) to prevent eye contact; and chemical resistant gloves and appropriate clothing (such as long-sleeve shirt and pants) to prevent skin contact and/or drug absorption. Wash hands after use.
In case of accidental eye contact, flush eyes immediately with water and seek medical attention. If wearing contact lenses, flush eyes immediately with water before removing lenses. In case of accidental skin contact and/or clothing contamination, wash skin thoroughly with soap and water and launder clothing with detergent. In case of ingestion do not induce vomiting and seek medical attention immediately. Probable mucosal damage may contraindicate the use of gastric lavage. Provide product label and/or package insert to medical personnel.
-
RESIDUE WARNING
RESIDUE WARNINGS: Cattle must not be slaughtered for human consumption within 8 days of the last treatment. Not for use in female dairy cattle 20 months of age or older, including dry dairy cows; use in these cattle may cause drug residues in milk and/or in calves born to these cows or heifers. Not for use in suckling beef calves, dairy calves, and veal calves. A withdrawal period has not been established for this product in pre-ruminating calves.
-
PRECAUTIONS
PRECAUTIONS: As a class, cyclo-oxygenase inhibitory NSAIDs may be associated with gastrointestinal, renal, and hepatic toxicity. Sensitivity to drug-associated adverse events varies with the individual patient. Patients at greatest risk for adverse events are those that are dehydrated, on concomitant diuretic therapy, or those with renal, cardiovascular, and/or hepatic dysfunction. Banamine transdermal should be used with caution in animals with suspected pre-existing gastric erosions or ulcerations. Concurrent administration of other NSAIDs, corticosteroids, or potentially nephrotoxic drugs should be avoided or used only with careful monitoring because of the potential increase of adverse events.
NSAIDs are known to have potential effects on both parturition (see Contraindications) and the estrous cycle. There may be a delay in the onset of estrus if flunixin is administered during the prostaglandin phase of the estrous cycle. NSAIDs are known to have the potential to delay parturition through a tocolytic effect. The use of NSAIDs in the immediate post-partum period may interfere with uterine involution and expulsion of fetal membranes. Cows should be monitored carefully for placental retention and metritis if Banamine Transdermal pour-on is used within 24 hours after parturition.
Not for use in dairy or beef bulls intended for breeding because reproductive safety has not been evaluated.
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY: Flunixin meglumine is a nonsteroidal, anti-inflammatory drug. It is a weak acid (pKa=5.82)1 which exhibits a high degree of plasma protein binding (approximately 99%).2 However, free (unbound) drug appears to readily partition into body tissues (Vss predictions range from 297 to 782 mL/kg).2-5 In cattle, elimination occurs primarily through biliary excretion.
Flunixin persists in inflammatory tissues6 and is associated with anti-inflammatory properties which extend well beyond the period associated with detectable plasma drug concentrations.4,6 Therefore, prediction of drug concentrations based upon the estimated plasma terminal elimination half-life will likely underestimate both the duration of drug action and the concentration of drug remaining at the site of activity.
Pharmacokinetic properties of flunixin transdermal solution in cattle administered at a dose of 2.5 mg/kg, are summarized in Table 1, comparing results between animals that were allowed to self- and allo-lick vs. animals that were prevented from licking. Animals that were allowed to self- or allo-lick had lower rate and extent of absorption when compared to the animals prevented from licking. However, no dose adjustment is needed to account for the effect of licking because the substantial evidence of effectiveness was demonstrated in animals that were allowed to lick.
Table 1. Average (+/- standard deviation [SD]) PK parameters after a single administration of flunixin transdermal solution at a dose of 2.5 mg/kg in cattle that were either allowed to lick or prevented from allo- and self-licking (n = 24/group). PK parameter Non-licking Licking Mean ± SD Mean ± SD Cmax: Maximum observed plasma concentration
Tmax: Time at which Cmax was observed
AUC2-last: Area under the plasma concentration versus time curve measured between 2 hours and the time of the last quantifiable concentration
T1/2: Terminal elimination half-life- * First blood level in the licking group was taken at 2 hours post-dose.
First blood sample in non-licking group was taken at 0.25 hours post-dose.Cmax (ng/mL) 1496 769 N/A N/A Concentration at 2 h* 1282 533 1072 353 Tmax (h) 1.29 0.464 N/A N/A AUC2-last (ng*h/mL) 7499 2131 6827 4672 T1/2 (h) 8 2 9 6 Absorption of flunixin transdermal solution in cattle is dependent on environmental temperature. The effect of temperature on flunixin absorption was tested in temperatures ranging from 15.3 to 20.1 °F (average low in the coldest study) to 80 to 100 °F (average high in the warmest study). Flunixin concentrations were consistently lower when the pour-on product was administered in a cold (temperature) rather than hot (temperature) environment. However, the clinical effectiveness was demonstrated over the range of environmental conditions expected under field conditions. No dose adjustments are necessary due to environmental temperature.
- * First blood level in the licking group was taken at 2 hours post-dose.
-
References:
1. Johansson M, Anler EL. Gas chromatographic analysis of flunixin in equine urine after extractive methylation. J Chromatogr. 1988; 427:55-66.
2. Odensvik K, Johansson M. High-performance liquid chromatography method for determination of flunixin in bovine plasma and pharmacokinetics after single and repeated doses of the drug. Am J Vet Res. 1995; 56:489-495.
3. Anderson KL, Neff-Davis CA, Davis LE, Bass VD. Pharmacokinetics of flunixin meglumine in lactating cattle after single and multiple intramuscular and intravenous administrations. Am J Vet Res. 1990; 51:1464-1467.
4. Odensvik K. Pharmacokinetics of flunixin and its effect on prostaglandin F2α metabolite concentrations after oral and intravenous administration in heifers. J Vet Pharmacol Ther. 1995;18:254-259.
5. Hardee GE, Smith JA, Harris SJ. Pharmacokinetics of flunixin meglumine in the cow. Res Vet Sci. 1985; 39:110-112.
6. Lees P, Higgins AJ. Flunixin inhibits prostaglandin E2 production in equine inflammation. Res Vet Sci. 1984; 37:347-349.
-
SPL UNCLASSIFIED SECTION
TARGET ANIMAL SAFETY: In a target animal safety study in 32 six-month old beef cattle (16 castrated males and 16 females), flunixin transdermal solution was administered topically at 3.3, 9.9, and 16.5 mg/kg body weight (1×, 3×, and 5× the labeled dose) on Days 1, 2, and 3 (3× the labeled administration frequency). Cattle were continuously restrained to prevent licking. In addition, the study was conducted under warm environmental conditions (70 °F to 80 °F on dosing days). One animal in the 3× group and three animals in the 5× group exhibiting twisting, kicking, rubbing on the fence, and or prancing, starting 5 to 15 minutes after dosing and lasting up to an hour after dosing on both Days 2 and 3. Two 5× animals had positive fecal occult blood on one of three post-treatment days, and one 5× animal had positive fecal occult blood on Days 2 and 3 post-treatment. Trace occult blood was found in the urine of three animals: one 5× animal on Day 1, one 5× animal on Day 3, and one 3× animal on Day 3. Test article-related pathology changes included a dose-related increase in the incidence and severity of abomasal erosions and ulcerations; and inflammatory cell infiltrates, epidermal necrosis, and small areas of dermal necrosis at the application site. The abomasal lesions correlated with fecal occult blood in three 5× animals. There were no animals with any other evidence of gastrointestinal bleeding or clinical signs of abomasal ulceration during the study.
Application site reactions, including dandruff/skin flakes, hair damage (thin, broken, brittle hair), and skin thickening were observed in effectiveness and/or supportive studies. The application site reactions were first observed around three to seven days post-dosing and lasted for about 14 days. These reactions were cosmetic in nature and generally resolved without treatment.
A pharmacokinetic evaluation demonstrated that the systemic exposure of flunixin is markedly lower when administered transdermally at a dose of 3.3 mg flunixin/kg BW than when administered intravenously at a dose of 2.2 mg flunixin/kg BW, therefore, female reproductive safety is supported by reproductive safety studies conducted for the approval of BANAMINE (flunixin meglumine injection) in cattle, NADA 101-479.
-
SPL UNCLASSIFIED SECTION
EFFECTIVENESS: Pharmacokinetic studies established that the absorption of flunixin administered transdermally to cattle is highly dependent on environmental temperature. Therefore the effectiveness of flunixin transdermal solution for the control of pyrexia associated with bovine respiratory disease was demonstrated under a range of environmental temperatures in two studies: a field study conducted at four geographic locations (California, Kansas, Nebraska, and Texas) under moderate environmental temperatures (average temperatures ranged from 42 °F to 74 °F on enrollment days) and a field study conducted at a single site (Nebraska) under cold environmental conditions (average temperatures ranged from 2 °F to 20 °F on enrollment days). In both studies, cattle were housed in groups and were not prevented from licking.
In both studies, cattle exhibiting clinical signs of BRD and having a rectal temperature of at least 104.5 °F were enrolled. A total of 235 cattle in the multi-location field study and 50 cattle at the single site field study were administered either flunixin transdermal solution (3.3 mg/kg BW) or an equivalent volume of dyed saline as a pour-on once on Day 0. Six hours after treatment, rectal temperatures were measured. The treatment success rate of the flunixin transdermal solution-treated group was compared to the treatment success rate in the dyed saline-treated group. A treatment success was defined as a drop in rectal temperature of ≥ 2 °F in an individual animal. In the multi-location study, the treatment success rate was significantly different (p < 0.0001) and higher for the flunixin transdermal solution-treated group (70/120, 58.3%) compared to the dyed saline-treated control group (7/115, 6.1%). In the single site study, the treatment success rate was significantly different (p = 0.0002) and higher for the flunixin transdermal solution-treated group (19/25, 76%) compared to the dyed saline-treated control group (4/25, 16%).
The effectiveness of flunixin transdermal solution for the control of pain associated with foot rot in beef and dairy cattle was demonstrated under a range of environmental temperatures in two studies: an induced infection model study conducted in Nebraska with temperatures ranging from 61 °F to 85 °F on the day of enrollment and treatment; and an induced infection model study conducted in Kansas with temperatures ranging from 27 °F to 53 °F on the day of enrollment and treatment. In both studies, cattle from both treatment groups were commingled in pens and were not prevented from licking.
In each study, cattle were challenged by subcutaneous injection of a culture of Fusobacterium necrophorum into the interdigital space of the right front foot using a method that was validated to induce pain representative of foot rot. Cattle were enrolled when they demonstrated signs of pain associated with foot rot based on lameness, interdigital lesion, and interdigital swelling criteria. Pressure mat gait parameters maximum total force (kgf) and contact area (cm2) were also measured at enrollment. A total of 30 cattle at each site were administered either flunixin transdermal solution (3.3 mg/kg BW) or an equivalent volume of dyed saline as a pour-on once on Day 0. Six hours after treatment, lameness scores and pressure mat gait parameters maximum total force and contact area were measured.
Effectiveness was determined independently at each site based on treatment success rates at six hours after treatment; and the change in maximum total force and contact area between enrollment and six hours after treatment. A treatment success was defined as a decrease in lameness score by ≥ 1 (scale 1 to 5, with enrollment of animals with lameness score ≥ 3) from the enrollment lameness score. The treatment success rate of the flunixin transdermal solution-treated group was compared to the treatment success rate in the dyed saline-treated group at both sites.
Changes in biometric gait parameters were also compared between the treatment groups.
In the Nebraska study, the treatment success rate was significantly different and higher for the flunixin transdermal solution-treated group (15/15, 100%) compared to the dyed saline-treated group (1/15, 6.67%); and the mean change in maximum total force and mean change in contact area were statistically significantly different (p <0.0001) and higher in the flunixin transdermal solution-treated group (43.08 kgf and 16.76 cm2) compared to the dyed saline-treated control group (-4.14 kgf and -2.70 cm2). In the Kansas study, the treatment success rate was significantly different (p=0.0387) and higher for the flunixin transdermal solution-treated group (14/15, 93.33%) compared to the dyed saline-treated group (8/15, 53.33%); and the mean change in maximum total force and mean change in contact area were statistically significantly different (p= 0.0002 and p<0.0001, respectively) and higher in the flunixin transdermal solution-treated group (34.32 kgf and 16.38 cm2) compared to the dyed saline-treated control group (-0.54 kgf and -0.96 cm2).
-
CONTACT INFORMATION:
For technical assistance or to report a suspected adverse drug experience, call: 1-800-219-9286
For customer service or to request a Safety Data Sheet (SDS), call: 1-800-211-3573.
For additional Banamine Transdermal pour-on information go to www.BanamineTD.com
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/AnimalVeterinary/SafetyHealth."
-
HOW SUPPLIED
HOW SUPPLIED: Banamine Transdermal pour-on, is available in 100-mL (NDC: 0061-4363-01), 250-mL (NDC: 0061-4363-02), and 1-L (NDC: 0061-4363-03) bottles.
-
SPL UNCLASSIFIED SECTION
For Patent information: http://www.merck.com/product/patent/home.html
NADA 141-450, Approved by FDA
Use Only as Directed
Copyright © 2017, Intervet Inc., a subsidiary of Merck & Co.
All rights reserved.
Made in Germany
Distributed by: Intervet Inc. d/b/a Merck Animal Health, Madison, NJ 079405/2017
196764 R1
-
PRINCIPAL DISPLAY PANEL - 250 mL Bottle Carton
NDC: 0061-4363-02
Multiple-Dose BottleBanamine®
Transdermal
(flunixin transdermal
solution)Pour-On for Beef and Dairy Cattle
50 mg/mLNon-Steroidal Anti-inflammatory Drug
INDICATIONS: Banamine Transdermal pour-on is indicated
for the control of pyrexia associated with bovine respiratory
disease and the control of pain associated with foot rot
in steers, beef heifers, beef cows, beef bulls intended for
slaughter, and replacement dairy heifers under 20 months
of age. Not for use in beef bulls intended for breeding; dairy
bulls; female dairy cattle 20 months of age or older, including
dry dairy cows; and suckling beef calves, dairy calves, and veal
calves.CAUTION: Federal law
restricts this drug to use
by or on the order of a
licensed veterinarianNADA 141-450
Approved by FDANET CONTENTS: 250 mL
MERCK
Animal Health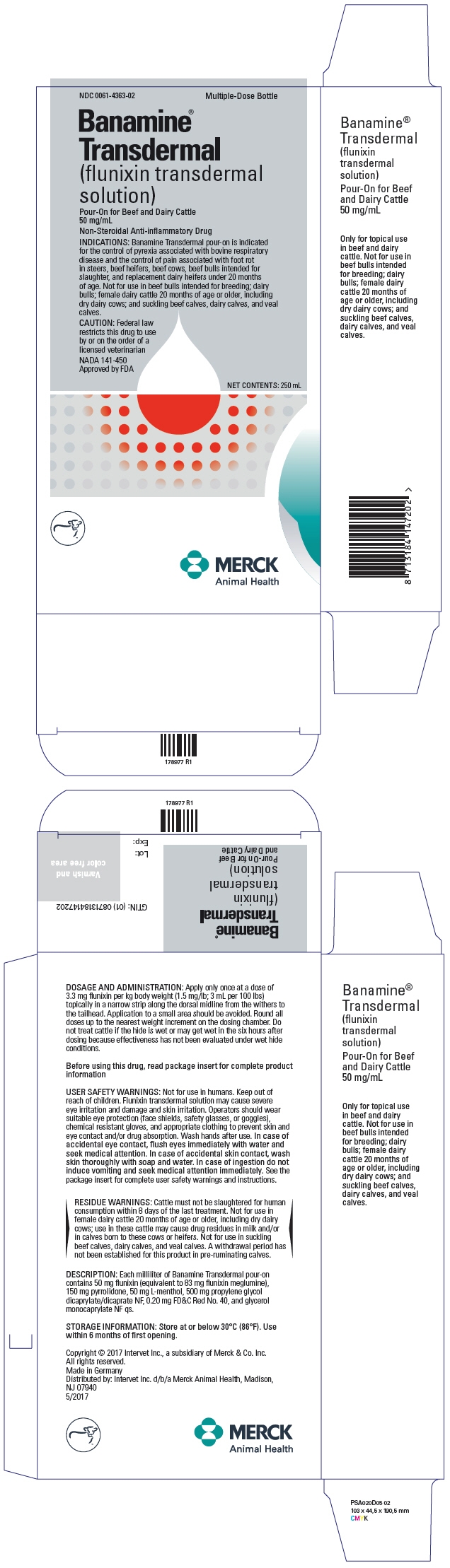
-
INGREDIENTS AND APPEARANCE
BANAMINE
flunixin meglumine solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0061-4363 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Flunixin Meglumine (UNII: 8Y3JK0JW3U) (Flunixin - UNII:356IB1O400) Flunixin 50 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0061-4363-01 1 in 1 CARTON 1 100 mL in 1 BOTTLE, PLASTIC 2 NDC: 0061-4363-02 1 in 1 CARTON 2 250 mL in 1 BOTTLE, PLASTIC 3 NDC: 0061-4363-03 1 in 1 CARTON 3 1000 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141450 01/31/2018 Labeler - Merck Sharp & Dohme Corp. (001317601) Establishment Name Address ID/FEI Business Operations Vet Pharma Friesoythe GmbH 341934053 MANUFACTURE
Trademark Results [Banamine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BANAMINE 73058123 1032757 Live/Registered |
SCHERING CORPORTION 1975-07-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.